HPLC-DAD vs. UHPLC-UV for Posaconazole Quantitation: A Comprehensive Guide for Method Development and Validation
This article provides a systematic comparison of High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) and Ultra-High-Performance Liquid Chromatography with Ultraviolet Detection (UHPLC-UV) for the quantitation of posaconazole in bulk,...
HPLC-DAD vs. UHPLC-UV for Posaconazole Quantitation: A Comprehensive Guide for Method Development and Validation
Abstract
This article provides a systematic comparison of High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) and Ultra-High-Performance Liquid Chromatography with Ultraviolet Detection (UHPLC-UV) for the quantitation of posaconazole in bulk, pharmaceutical formulations, and biological samples. Tailored for researchers and drug development professionals, the content explores the foundational principles, detailed methodologies, and optimization strategies for both techniques. It further delivers a critical validation framework based on International Conference on Harmonisation (ICH) guidelines, enabling informed selection and implementation of these analytical methods for quality control, pharmacokinetic studies, and clinical research.
Understanding Posaconazole and the Principles of HPLC-DAD and UHPLC-UV Analysis
The Critical Role of Posaconazole Quantitation in Antifungal Therapy and Quality Control
Posaconazole (PSZ) is a broad-spectrum triazole antifungal agent crucial for treating and preventing invasive fungal infections in immunocompromised patients, including those with hematopoietic stem cell transplants, hematologic malignancies, or HIV/AIDS [1]. The quantitation of posaconazole in pharmaceutical formulations and biological matrices is essential for ensuring therapeutic efficacy, patient safety, and product quality. This application note explores advanced analytical techniques for posaconazole quantification, focusing specifically on the comparative merits of High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) and Ultra-High Performance Liquid Chromatography with Ultraviolet Detection (UHPLC-UV) within the context of pharmaceutical quality control and clinical monitoring.
The critical importance of accurate posaconazole quantification stems from its narrow therapeutic index and the serious consequences of subtherapeutic or toxic concentrations. Efficient analytical methods are required for various applications, including drug development, quality control of pharmaceutical products, therapeutic drug monitoring in clinical settings, and pharmacokinetic studies [2] [3]. This document provides detailed protocols, comparative data, and technical guidance to support researchers and analysts in implementing robust posaconazole quantification methods.
Analytical Technique Comparison: HPLC-DAD vs. UHPLC-UV
The selection of an appropriate analytical technique is fundamental to successful posaconazole quantification. HPLC-DAD and UHPLC-UV represent two prominent approaches with distinct characteristics and advantages.
Table 1: Comparison of HPLC-DAD and UHPLC-UV Methods for Posaconazole Quantitation
| Parameter | HPLC-DAD Method | UHPLC-UV Method |
|---|---|---|
| Stationary Phase | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) [1] | Kinetex-C18 (2.1 × 50 mm, 1.3 μm) [1] |
| Mobile Phase | Gradient: Acetonitrile:15 mM KH₂PO₄ (30:70 to 80:20) [1] | Isocratic: Acetonitrile:15 mM KH₂PO₄ (45:55) [1] |
| Flow Rate | 1.5 mL/min [1] | 0.4 mL/min [1] |
| Run Time | 11 minutes [1] | 3 minutes [1] |
| Injection Volume | 20-50 μL [1] | 5 μL [1] |
| Linear Range | 5-50 μg/mL [1] | 5-50 μg/mL [1] |
| Limit of Detection | 0.82 μg/mL [1] | 1.04 μg/mL [1] |
| Limit of Quantification | 2.73 μg/mL [1] | 3.16 μg/mL [1] |
| Key Advantages | Robustness, wider availability | Faster analysis, reduced solvent consumption, superior separation efficiency |
The fundamental difference between these techniques lies in the particle size of the stationary phase, with UHPLC utilizing sub-2μm particles to achieve enhanced efficiency. This allows UHPLC to operate at higher pressures with mobile phases running at greater linear velocities compared to conventional HPLC, resulting in significant reductions in analytical time, sample volume, and solvent consumption while providing superior chromatographic separation [1].
Advanced Method Optimization Approaches
Recent research has introduced sophisticated optimization strategies for posaconazole quantification methods. One innovative approach combines a 2-level fractional factorial design with machine learning models, including Artificial Neural Networks (ANN) and Genetic Algorithms (GA), to optimize both chromatographic and extraction parameters simultaneously [3]. This method allows for the development of rapid assays with low limits of quantification (50 ng/mL) in low-volume plasma samples (100 μL), which is particularly valuable in preclinical pharmacokinetic studies involving small animals [3].
Table 2: Validation Parameters for Posaconazole HPLC Methods
| Validation Parameter | HPLC-DAD Performance [1] | Recent HPLC-UV Performance [2] |
|---|---|---|
| Linearity (Range) | 5-50 μg/mL (r² > 0.999) [1] | 2-20 μg/mL [2] |
| Precision (CV%) | <3% [1] | <1% [2] |
| Accuracy (% Error) | <3% [1] | ~99% Recovery [2] |
| Specificity | No observable interferences from suspension excipients [1] | No interference from diluents or excipients [2] |
Experimental Protocols
HPLC-DAD Method for Posaconazole Quantification in Suspension Dosage Form
Principle: This method utilizes reversed-phase chromatography with gradient elution and diode array detection for the quantification of posaconazole in oral suspension formulations [1].
Materials and Reagents:
- Posaconazole reference standard
- Itraconazole (Internal Standard)
- HPLC grade methanol and acetonitrile
- Analytical grade potassium dihydrogen orthophosphate
- High purity distilled water
- Oral suspension (40 mg/mL)
Equipment:
- HPLC system with quaternary pump, degasser, and diode array detector
- Zorbax SB-C18 column (4.6 × 250 mm, 5 μm)
- Analytical balance
- pH meter
- Vortex mixer
- Centrifuge
Mobile Phase Preparation: Prepare 15 mM potassium dihydrogen orthophosphate buffer. Filter through a 0.45 μm membrane filter. The gradient program consists of acetonitrile and phosphate buffer in a ratio changing from 30:70 to 80:20 linearly over 7 minutes [1].
Standard Solution Preparation:
- Prepare a 100 μg/mL stock solution of posaconazole by dissolving 10 mg in 100 mL methanol.
- Prepare a 100 μg/mL stock solution of itraconazole (IS) by dissolving 10 mg in 100 mL methanol.
- Prepare working solutions by appropriate dilution with methanol to concentrations ranging from 5-50 μg/mL.
- Add 10 μg/mL IS to each standard solution [1].
Sample Preparation:
- Dilute 0.1 mL of oral suspension (40 mg/mL) to 10 mL with methanol (S1).
- Add 10 μg/mL IS to 0.1 mL of S1 supernatant and dilute with methanol to a final volume of 1 mL (S2).
- Vortex mix for 10 seconds at high speed.
- Centrifuge if necessary to remove particulates [1].
Chromatographic Conditions:
- Column: Zorbax SB-C18 (4.6 × 250 mm, 5 μm)
- Mobile Phase: Gradient of acetonitrile and 15 mM potassium dihydrogen orthophosphate
- Flow Rate: 1.5 mL/min
- Injection Volume: 20-50 μL
- Column Temperature: 25°C
- Detection Wavelength: 262 nm
- Run Time: 11 minutes [1]
Procedure:
- Equilibrate the column with initial mobile phase composition for at least 30 minutes.
- Inject blank (methanol), standard solutions, and prepared samples.
- Run the gradient program and record chromatograms.
- Measure peak areas of posaconazole and internal standard.
- Construct calibration curve by plotting peak area ratio (posaconazole/IS) versus concentration.
- Calculate posaconazole concentration in unknown samples using the regression equation.
UHPLC-UV Method for Posaconazole Quantification
Principle: This method utilizes ultra-high performance liquid chromatography with isocratic elution and UV detection for rapid quantification of posaconazole [1].
Materials and Reagents:
- Similar to HPLC-DAD method with adjustments for UHPLC compatibility
Equipment:
- UHPLC system with binary pump and UV detector
- Kinetex-C18 column (2.1 × 50 mm, 1.3 μm)
Mobile Phase Preparation: Prepare a mixture of acetonitrile and 15 mM potassium dihydrogen orthophosphate in the ratio 45:55. Filter through 0.22 μm membrane filter and degas [1].
Standard and Sample Preparation: Follow similar procedures as the HPLC-DAD method, with appropriate adjustment of concentrations for the lower injection volume.
Chromatographic Conditions:
- Column: Kinetex-C18 (2.1 × 50 mm, 1.3 μm)
- Mobile Phase: Isocratic acetonitrile:15 mM potassium dihydrogen orthophosphate (45:55)
- Flow Rate: 0.4 mL/min
- Injection Volume: 5 μL
- Column Temperature: 40°C
- Detection Wavelength: 262 nm
- Run Time: 3 minutes [1]
Procedure:
- Equilibrate the UHPLC system with mobile phase.
- Inject blank, standards, and samples.
- Record chromatograms and measure peak areas.
- Construct calibration curve and calculate concentrations as described in the HPLC-DAD protocol.
Method Validation Protocols
Both methods should be validated according to International Conference on Harmonisation (ICH) guidelines [1] [2]. The following validation parameters should be assessed:
Linearity: Prepare and analyze at least five concentrations over the claimed range (e.g., 5-50 μg/mL). The correlation coefficient (r²) should be greater than 0.999 [1].
Precision: Assess intra-day precision using three replicates of three different concentrations (e.g., 5, 20, and 50 μg/mL) analyzed on the same day. Determine inter-day precision by analyzing the same concentrations on three separate days. The coefficient of variation (CV%) should be less than 3% [1] or 1% [2] depending on the method.
Accuracy: Determine recovery by analyzing samples of known concentrations and calculating the percentage error. The mean percentage error should be less than 3% [1].
Specificity: Verify that excipients or other components in the sample do not interfere with the posaconazole peak. Analyze blank samples and samples spiked with excipients [1] [2].
Limits of Detection and Quantification: Determine LOD and LOQ based on signal-to-noise ratios of 3:1 and 10:1, respectively [1].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents and Materials for Posaconazole Quantitation
| Item | Specification/Function | Application Examples |
|---|---|---|
| Stationary Phases | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) for HPLC; Kinetex-C18 (2.1 × 50 mm, 1.3 μm) for UHPLC [1] | Reversed-phase separation of posaconazole [1] |
| Mobile Phase Components | Acetonitrile (organic modifier); Potassium dihydrogen orthophosphate (buffer) [1] | Creating appropriate elution strength and controlling pH [1] |
| Internal Standard | Itraconazole (structurally related azole antifungal) [1] | Normalizing variations in extraction and injection [1] |
| Extraction Solvents | Diethyl ether, tertiary butyl methyl ether (TBME) [3] [4] | Liquid-liquid extraction of posaconazole from plasma samples [3] |
| Reference Standards | Posaconazole (purity >99%) [1] [4] | Preparation of calibration standards and quality control samples [1] |
Advanced Applications and Specialized Techniques
Polymorph Quantitation in Oral Suspensions
Posaconazole exhibits extensive polymorphism, with at least fourteen different forms reported, including ten crystal polymorphs, three solvates, and an amorphous form [5]. In oral suspensions, Form I is typically used as the raw material due to its stability. However, a polymorph transition from Form I to a hydrated Form-S has been observed in commercial oral suspensions [5].
Raman spectroscopy has been successfully employed for the simultaneous quantitation of posaconazole Form I and Form-S in oral suspensions, addressing challenges such as polymorph instability and preferred orientation issues associated with X-ray powder diffraction (XRPD) [5]. This technique, combined with a specialized rotary apparatus and fiber-coupled Raman trigger probe, effectively eliminates issues of dose inhomogeneity in these complex formulations [5].
Simultaneous Drug Quantification for Interaction Studies
Analytical methods have been developed for the simultaneous determination of posaconazole and other drugs, such as vincristine, in biological matrices. These methods are particularly valuable for drug-drug interaction studies, allowing pharmacokinetic analysis using a single blood sample for multiple drugs without the need for sample splitting [4]. One validated HPLC-DAD method successfully quantified both posaconazole and vincristine in rat plasma over a range of 50-5000 ng/mL, with a total run time of 11 minutes [4].
Bioanalytical Applications and Plasma Quantification
Recent advances in bioanalytical methods have focused on quantifying posaconazole in low-volume plasma samples, essential for preclinical pharmacokinetic studies in small animals. One novel approach combining experimental design and machine learning achieved a quantification limit of 50 ng/mL using only 100 μL of plasma [3]. The optimized method employed liquid-liquid extraction with high recovery rates (>98%) and a chromatographic run time of 8.2 minutes, significantly improving efficiency for high-throughput analyses [3].
The accurate quantification of posaconazole remains a critical component in ensuring the efficacy and safety of this important antifungal medication. HPLC-DAD and UHPLC-UV methods both provide valid, reliable approaches for posaconazole quantitation in pharmaceutical formulations and biological samples, with the choice depending on specific application requirements.
UHPLC-UV offers distinct advantages in analysis speed, solvent consumption, and separation efficiency, making it particularly suitable for high-throughput quality control environments. HPLC-DAD remains a robust, widely accessible alternative with excellent performance characteristics. For specialized applications such as polymorph quantification or therapeutic drug monitoring, additional techniques including Raman spectroscopy or optimized extraction protocols may be employed.
The continued refinement of posaconazole quantification methods through advanced approaches such as experimental design and machine learning optimization promises further enhancements in sensitivity, efficiency, and application scope, ultimately contributing to improved patient outcomes through better product quality and therapeutic monitoring.
The quantitative analysis of active pharmaceutical ingredients (APIs), such as the antifungal drug posaconazole, is a critical requirement in pharmaceutical development and quality control. Selecting the appropriate analytical technique is paramount for achieving accurate, reproducible, and efficient results. Two prominent liquid chromatography platforms used for this purpose are High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) and Ultra-High-Performance Liquid Chromatography with UV Detection (UHPLC-UV). The fundamental distinction between a DAD and a standard UV detector lies in their optical design; a variable wavelength UV detector selects a specific wavelength to pass through the sample flow cell, whereas a DAD passes the entire light spectrum through the flow cell and then disperses it onto an array of diodes, capturing the full absorbance spectrum simultaneously [6] [7]. This application note, framed within a broader research thesis on posaconazole quantitation, provides a detailed comparison of HPLC-DAD and UHPLC-UV methodologies. We summarize key performance data in structured tables, outline detailed experimental protocols for replicating the analysis, and visualize the system configurations to guide scientists in selecting the optimal technique for their specific application needs.
Core Principles and Instrumentation
Detection: Diode Array Detection (DAD) vs. Ultraviolet Detection (UV)
The detection mechanism is a primary differentiator between these two techniques. A Diode Array Detector (DAD), also known as a Photodiode Array (PDA), is characterized by its ability to capture the complete ultraviolet and visible (UV-Vis) absorption spectrum of an analyte as it elutes from the column. This is achieved by passing polychromatic light through the sample flow cell, after which the transmitted light is dispersed by a diffraction grating onto an array of photodiodes [8] [6]. This design allows for the collection of data across a range of wavelengths simultaneously for every data point in the chromatogram.
Key advantages of DAD include:
- Peak Purity Analysis: The acquired spectral data enables scientists to confirm the homogeneity of a chromatographic peak by comparing spectra from different points across the peak (e.g., the upslope, apex, and downslope) [6] [7].
- Spectral Identification: Unknown peaks can be tentatively identified by matching their absorption spectrum against a library of reference spectra [7].
- Method Development Flexibility: It allows for the retrospective selection of the optimal wavelength for quantification without reinjecting the sample.
In contrast, a variable wavelength UV (or UV-Vis) detector operates by selecting a single, specific wavelength from the light source using a diffraction grating. This monochromatic light is then passed through the flow cell where the sample absorbs a portion of it [6] [7]. While this design can offer marginally higher sensitivity for that specific wavelength due to reduced optical complexity, it provides no spectral information beyond the absorbance at the preset wavelength.
Separation: HPLC vs. UHPLC
The separation power of UHPLC primarily stems from the use of chromatographic columns packed with smaller particles, typically less than 2 µm in diameter. According to the van Deemter equation, smaller particles provide higher chromatographic efficiency (theoretical plates, N), which translates to sharper peaks and greater resolution [9]. To utilize these columns, UHPLC systems are engineered to operate at significantly higher pressures (exceeding 15,000 psi or 1000 bar) compared to conventional HPLC systems (typically up to 6,000 psi or 400 bar) [1] [10]. The benefits of this fundamental advancement are multi-fold:
- Enhanced Speed: The higher efficiency allows for faster separations without sacrificing resolution, as shorter columns can be used to achieve the same separation in a fraction of the time [9].
- Improved Resolution: The increased efficiency per unit column length enables the separation of complex mixtures with very similar compounds, including isomers and impurities [9].
- Increased Sensitivity: Sharper peaks result in higher peak concentrations, which improves the signal-to-noise ratio for detection [9] [11].
- Reduced Solvent Consumption: Faster run times and the use of narrower-bore columns lead to a dramatic reduction in solvent usage, making UHPLC a "greener" alternative [9].
Comparative Analysis: HPLC-DAD vs. UHPLC-UV for Posaconazole
A direct comparative study of HPLC-DAD and UHPLC-UV for the quantitation of posaconazole (PSZ) in bulk powder and suspension dosage form provides a clear, data-driven perspective on their performance [1]. The table below summarizes the key experimental parameters and validation data from this study, offering a side-by-side comparison.
Table 1: Summary of Experimental Parameters for Posaconazole Quantitation
| Parameter | HPLC-DAD Method | UHPLC-UV Method |
|---|---|---|
| Column | Zorbax SB-C18 (4.6 × 250 mm, 5 µm) | Kinetex-C18 (2.1 × 50 mm, 1.3 µm) |
| Mobile Phase | Gradient: Acetonitrile:15 mM KH₂PO₄ (30:70 to 80:20) | Isocratic: Acetonitrile:15 mM KH₂PO₄ (45:55) |
| Flow Rate | 1.5 mL/min | 0.4 mL/min |
| Injection Volume | 20-50 µL | 5 µL |
| Run Time | 11 minutes | 3 minutes |
| Detection | DAD, 262 nm | UV, 262 nm |
Table 2: Method Validation Data Comparison
| Validation Parameter | HPLC-DAD Method | UHPLC-UV Method |
|---|---|---|
| Linearity Range | 5–50 µg/mL | 5–50 µg/mL |
| Correlation Coefficient (r²) | > 0.999 | > 0.999 |
| Limit of Detection (LOD) | 0.82 µg/mL | 1.04 µg/mL |
| Limit of Quantitation (LOQ) | 2.73 µg/mL | 3.16 µg/mL |
| Precision (CV%) & Accuracy (% Error) | < 3% | < 3% |
Interpretation of Comparative Data
The data reveals distinct advantages and trade-offs:
- Speed: The UHPLC-UV method demonstrates a clear superiority in analysis speed, completing a run in approximately 3 minutes compared to 11 minutes for the HPLC-DAD method—a nearly 4-fold increase in throughput [1].
- Separation Efficiency: The UHPLC method achieved excellent separation using a short column with small (1.3 µm) particles and an isocratic elution, whereas the HPLC method required a longer column with larger (5 µm) particles and a gradient elution profile [1].
- Sensitivity: In this specific study, the HPLC-DAD method showed slightly better LOD and LOQ values [1]. However, UHPLC is generally recognized for providing enhanced sensitivity due to sharper peak profiles. This particular result may be attributed to specific method optimization choices.
- Solvent Consumption: The UHPLC method used a lower flow rate (0.4 mL/min vs. 1.5 mL/min), resulting in significantly lower solvent consumption per run, aligning with its "greener" profile [1] [9].
Experimental Protocols
Detailed Protocol: Quantitation of Posaconazole by UHPLC-UV
This protocol is adapted from the comparative study and is designed for the determination of posaconazole in a suspension dosage form [1].
I. Materials and Reagents
- Analytical Standards: Posaconazole (PSZ) and Itraconazole (Internal Standard, IS).
- Solvents: HPLC-grade methanol and acetonitrile.
- Reagents: Analytical grade potassium dihydrogen orthophosphate (KH₂PO₄).
- Samples: Posaconazole oral suspension (e.g., Noxafil 40 mg/mL).
- Equipment: UHPLC system capable of high-pressure operation (e.g., Agilent 1290 Infinity), binary pump, autosampler, column oven, and UV detector. Kinetex-C18 (2.1 × 50 mm, 1.3 µm) or equivalent column.
II. Preparation of Solutions
- Stock Solution of PSZ (100 µg/mL): Accurately weigh 10 mg of PSZ reference standard and transfer to a 100 mL volumetric flask. Dissolve and make up to volume with methanol.
- Stock Solution of IS (100 µg/mL): Accurately weigh 10 mg of Itraconazole and transfer to a 100 mL volumetric flask. Dissolve and make up to volume with methanol.
- Mobile Phase (15 mM KH₂PO₃ Buffer:ACN, 55:45): Dissolve 2.04 g of KH₂PO₄ in 1 L of high-purity water. Filter through a 0.45 µm membrane filter. Mix 550 mL of this buffer with 450 mL of HPLC-grade acetonitrile. Degas the mobile phase before use.
- Calibration Standards: Prepare working solutions from the PSZ stock by dilution with methanol to concentrations spanning the 5–50 µg/mL range. To each calibration standard, add a fixed volume of the IS working solution (e.g., to achieve 10 µg/mL final concentration) and dilute to the final volume with methanol.
III. UHPLC-UV Instrumental Conditions
- Column: Kinetex-C18 (2.1 × 50 mm, 1.3 µm)
- Column Temperature: 40 °C
- Mobile Phase: Acetonitrile: 15 mM Potassium Dihydrogen Orthophosphate (45:55, v/v)
- Flow Rate: 0.4 mL/min
- Injection Volume: 5 µL
- Detection Wavelength: 262 nm
- Run Time: 3 minutes
IV. Sample Preparation
- Pipette 0.1 mL of the posaconazole oral suspension into a 10 mL volumetric flask. Dilute to volume with methanol and mix thoroughly (Solution S1).
- Transfer a 0.1 mL aliquot of S1 to a 2.5 mL microcentrifuge tube.
- Add a fixed volume of the IS working solution (e.g., 10 µg/mL) to the tube.
- Dilute the mixture to 1 mL with methanol and vortex for 10-15 seconds.
- Centrifuge if necessary, and transfer the supernatant to an HPLC vial for analysis.
V. Validation Parameters The method should be validated according to ICH guidelines [1] [12]. Assess the following:
- Linearity: Analyze calibration standards in triplicate across the 5–50 µg/mL range.
- Precision and Accuracy: Determine intra-day and inter-day precision (CV%) and accuracy (% bias) using QC samples at low, medium, and high concentrations within the range.
- Specificity: Verify that the excipients in the suspension do not interfere with the PSZ or IS peaks.
- LOD and LOQ: Determine based on signal-to-noise ratios of 3:1 and 10:1, respectively.
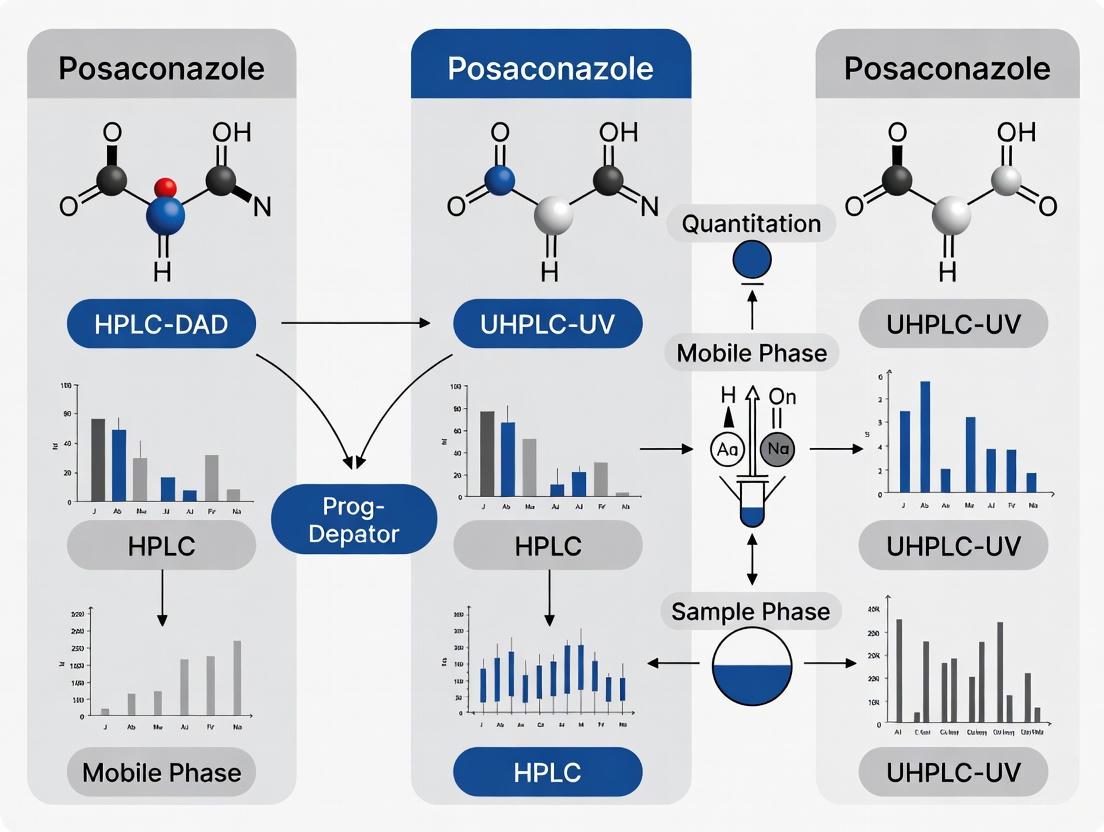
Workflow Visualization
The following diagram illustrates the logical workflow for the development and execution of the posaconazole quantitation method, highlighting the parallel paths for HPLC-DAD and UHPLC-UV.
Diagram 1: HPLC-DAD and UHPLC-UV Method Development Workflow for Posaconazole Quantitation
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table lists key materials and reagents required for setting up the posaconazole quantitation methods described in this note.
Table 3: Essential Research Reagent Solutions and Materials
| Item | Function / Application | Example / Specification |
|---|---|---|
| Posaconazole Reference Standard | Primary standard for calibration curve preparation; used to quantify the API in unknown samples. | Certified reference material of high purity (e.g., >98%). |
| Itraconazole | Internal Standard (IS); added in a fixed amount to all samples and standards to correct for injection volume variability and sample preparation losses. | Certified reference material of high purity. |
| HPLC-Grade Acetonitrile and Methanol | Organic solvent for stock solution preparation, sample extraction/dilution, and as a component of the mobile phase. | HPLC-grade, low UV absorbance. |
| Potassium Dihydrogen Phosphate (KH₂PO₄) | Buffer salt for mobile phase; helps control pH and improve chromatographic peak shape. | Analytical grade, ≥99.0%. |
| Ultrapure Water | Aqueous component of the mobile phase and for buffer preparation. | Resistivity of 18.2 MΩ·cm at 25°C, filtered through 0.45 µm or 0.22 µm membrane. |
| HPLC-DAD System | Instrument platform for HPLC-DAD analysis. | Includes quaternary pump, degasser, autosampler, column oven, and diode array detector. |
| UHPLC-UV System | Instrument platform for UHPLC-UV analysis. | Capable of operating at pressures >1000 bar (15,000 psi), with a binary pump and low-dispersion fluidics. |
| C18 Reverse-Phase Column | Stationary phase for chromatographic separation. | HPLC: Zorbax SB-C18 (4.6 x 250 mm, 5 µm). UHPLC: Kinetex-C18 (2.1 x 50 mm, 1.3 µm). |
The choice between HPLC-DAD and UHPLC-UV for the quantitation of posaconazole, or similar APIs, is not a matter of one being universally superior, but rather of selecting the right tool for the specific application requirements. UHPLC-UV offers compelling advantages in speed, solvent efficiency, and resolution power, making it ideal for high-throughput environments where rapid analysis times are critical. Conversely, HPLC-DAD provides the significant benefit of comprehensive spectral data, which is invaluable during method development, for confirming peak purity, and for identifying unknown impurities. The experimental protocols and data presented herein provide a robust foundation for scientists to implement and validate these methods, ensuring reliable and compliant analysis in pharmaceutical drug development and quality control.
Posaconazole is a broad-spectrum triazole antifungal agent critical for treating invasive fungal infections in immunocompromised patients. As a structural analogue of itraconazole, it demonstrates an extended spectrum of activity against most yeasts, filamentous fungi, and Candida species, including strains resistant to fluconazole. The quantitative analysis of posaconazole in pharmaceutical formulations requires precise understanding of its fundamental physicochemical and spectral properties. This application note details these essential characteristics within the broader research context comparing HPLC-DAD and UHPLC-UV quantification methodologies, providing researchers and drug development professionals with validated protocols for accurate analysis.
Physicochemical Properties
Posaconazole (chemical name: 4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)tetrahydro-5-(1H-1,2,4-triazol-1-ylmethyl)-3-furanyl]methoxy]phenyl]-1-piperazinyl]phenyl]-2-[(1S,2S)-1-ethyl-2-hydroxypropyl]-2,4-dihydro-3H-1,2,4-triazol-3-one) is a complex molecular entity with distinct physicochemical characteristics that influence its analytical behavior [13] [14].
Table 1: Fundamental Physicochemical Properties of Posaconazole
| Property | Value / Description | Reference |
|---|---|---|
| CAS Registry Number | 171228-49-2 | [15] |
| Molecular Formula | C({37})H({42})F({2})N({8})O(_{4}) | [15] [14] |
| Molecular Weight | 700.78 g/mol | [15] [14] |
| Melting Point | 170-172 °C | [15] |
| Optical Rotation | [α]/D -24 to -32° (c = 1.0 in chloroform-d) | [15] |
| pKa | 14.72 ± 0.20 (Predicted) | [15] |
| Boiling Point | 850.7 ± 75.0 °C (Predicted) | [15] |
| Density | 1.36 ± 0.1 g/cm³ (Predicted) | [15] |
| Appearance | White to beige powder | [15] |
Polymorphism and Hydrate Formation
A critical aspect of posaconazole's solid-state behavior is its polymorphism. At least fourteen different solid forms have been identified, including crystalline forms and solvates [16]. Form I is the thermodynamically stable polymorph used as the raw material for manufacturing oral suspensions. However, upon interaction with water during suspension formulation, a conversion to Form-S occurs [16]. This Form-S has been characterized as a trihydrate, incorporating three water molecules per API molecule, which can influence the dissolution rate and potentially the bioavailability of the final product [16]. The transformation is driven by the interaction with water, and complete conversion requires sonication to overcome the relatively poor wettability of Form I (contact angle of 75.3° ± 3.8°) [16].
Spectral Characteristics
Ultraviolet-Visible (UV-Vis) Spectroscopy
The UV spectrum of posaconazole is vital for its detection and quantification in chromatographic methods. The drug exhibits an absorbance maximum (λ_max) at 262 nm [1] [2]. This wavelength is consistently employed for UV detection in both HPLC and UHPLC assays. Theoretical studies using the CAM-B3LYP/6-31G(d,p) method have shown that the first excited state is connected to an electron excitation corresponding to a HOMO → LUMO+7 transition [13].
Vibrational Spectroscopy: IR and Raman
Experimental and theoretical analyses of the Fourier-Transform Infrared (FT-IR) spectrum have been performed. The geometry of posaconazole rotamers was optimized at the B3LYP/6-311++G(d,p) level of theory, showing good accordance with the experimental IR spectrum [13]. Key characteristic peaks in the experimental IR spectrum include:
- 3306 cm⁻¹: O-H stretching vibration
- 2967 cm⁻¹: C-H stretching vibration
- 1688 cm⁻¹: C=O stretching vibration
- 1614 cm⁻¹: C=C stretching of the aromatic ring
- 1394 cm⁻¹: C-N triazole or N=N stretching
- 1133 cm⁻¹: C-F stretching
- 965 cm⁻¹: C-O-C stretching [13]
Both Raman and Attenuated Total Reflection (ATR) spectroscopy are also effective for characterizing posaconazole and distinguishing between its different polymorphic forms, such as Form I and the hydrate Form-S [16].
Analytical Quantitation: HPLC-DAD vs. UHPLC-UV
The quantitation of posaconazole in bulk powder and suspension dosage forms has been successfully achieved using both HPLC-DAD and UHPLC-UV methods. The following table provides a direct comparison of two validated assays.
Table 2: Comparison of Validated HPLC-DAD and UHPLC-UV Methods for Posaconazole Quantitation
| Parameter | HPLC-DAD Method | UHPLC-UV Method | Reference |
|---|---|---|---|
| Analytical Column | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) | Kinetex-C18 (2.1 × 50 mm, 1.3 μm) | [1] |
| Mobile Phase | Gradient: ACN:15 mM KH₂PO₄ (30:70 to 80:20) | Isocratic: ACN:15 mM KH₂PO₄ (45:55) | [1] |
| Flow Rate | 1.5 mL/min | 0.4 mL/min | [1] |
| Injection Volume | 20-50 μL | 5 μL | [1] |
| Run Time | 11 minutes | 3 minutes | [1] |
| Detection Wavelength | 262 nm | 262 nm | [1] |
| Linearity Range | 5–50 μg/mL | 5–50 μg/mL | [1] |
| Correlation Coefficient (r²) | > 0.999 | > 0.999 | [1] |
| Limit of Detection (LOD) | 0.82 μg/mL | 1.04 μg/mL | [1] |
| Limit of Quantitation (LOQ) | 2.73 μg/mL | 3.16 μg/mL | [1] |
| Precision (CV%) | < 3% | < 3% | [1] |
Another developed HPLC method reported a linearity range of 2-20 μg/mL, with a percentage recovery of 99.01% from bulk and 99.05% from marketed formulations, and intra-day and inter-day precisions of less than 1% [2].
Experimental Protocols
Protocol 1: Sample Preparation for HPLC/UHPLC Analysis
This protocol describes the preparation of standard and sample solutions for the quantitation of posaconazole in suspension dosage forms [1].
- Standard Stock Solution (100 μg/mL): Accurately weigh 10 mg of posaconazole reference standard and transfer to a 100 mL volumetric flask. Dissolve and make up to volume with methanol.
- Internal Standard Solution (100 μg/mL): Accurately weigh 10 mg of itraconazole (IS) and transfer to a 100 mL volumetric flask. Dissolve and make up to volume with methanol.
- Calibration Curve Standards: From the stock solution, prepare working solutions by serial dilution with methanol to cover the concentration range of 5–50 μg/mL.
- Sample Preparation from Oral Suspension: a. Pipette 0.1 mL of the 40 mg/mL oral suspension into a 10 mL volumetric flask. b. Dilute to volume with methanol and mix well (Solution S1). c. Pipette 0.1 mL of S1 into a 2.5 mL microcentrifuge tube. d. Add a fixed volume of the 10 μg/mL IS working solution. e. Dilute to 1 mL with methanol and vortex mix for 10 seconds.
- Analysis: Inject the appropriate volume (20 μL for HPLC, 5 μL for UHPLC) into the chromatographic system.
Protocol 2: Isolation and Characterization of Posaconazole Hydrate (Form-S)
This protocol is used to investigate the polymorphic transformation of Posaconazole Form I to the hydrate Form-S in an aqueous environment [16].
- Dispersion: Prepare a 40 mg/mL aqueous dispersion of posaconazole Form I in purified water using magnetic stirring at 500 rpm for 30 minutes.
- Sonication: Subject the dispersion to sonication for at least 10 minutes to ensure complete wetting of the particles and facilitate complete polymorphic conversion.
- Isolation: Separate the solid phase from the dispersion either by: a. Centrifugation: Centrifuge at 8000 rpm, 25 °C for 23 minutes, or b. Filtration: Use vacuum filtration.
- Characterization: To prevent reversion to Form I, cover the isolated solid with a transparent low-density polyethylene (LDPE) film and characterize immediately. a. XRPD: Record the X-ray powder diffraction pattern. Characteristic peaks for Form-S appear at 10.2° and 24.6° 2-theta. b. Thermal Analysis: Perform TGA to confirm the weight loss corresponding to the three water molecules of the hydrate. c. Vibrational Spectroscopy: Use ATR or Raman spectroscopy to fingerprint the form.
Visualization of Workflows
Analytical Quantitation Workflow
Polymorphic Transformation Pathway
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for Posaconazole Analysis
| Item | Function / Role | Example / Specification |
|---|---|---|
| Posaconazole Reference Standard | Primary standard for calibration curve preparation. Purity ≥ 99% [13]. | |
| Itraconazole | Internal Standard (IS) to correct for volumetric and instrumental variability [1]. | |
| HPLC Grade Methanol & Acetonitrile | Solvent for preparing standard and sample solutions; component of the mobile phase. | |
| Potassium Dihydrogen Phosphate (KH₂PO₄) | Buffer component in the mobile phase to control pH and improve peak shape. | 15 mM, pH adjusted [1] [2] |
| C18 Reverse-Phase Chromatography Column | Stationary phase for analytical separation. | e.g., Zorbax SB-C18 (5 µm) for HPLC; Kinetex-C18 (1.3 µm) for UHPLC [1] |
| Syringe Filters | Clarification of samples prior to injection into the HPLC/UHPLC system. | 0.45 µm or 0.22 µm Nylon membrane [2] |
| Oral Suspension Formulation | Sample matrix for analysis. | Contains posaconazole (40 mg/mL), polysorbate 80, simethicone, xanthan gum, etc. [16] |
| Purified Water | Dispersion medium for polymorphic studies; component of aqueous mobile phase. |
In the pharmaceutical analysis of compounds like posaconazole, a broad-spectrum triazole antifungal agent, the selection of an optimal detection wavelength is a critical parameter that directly impacts method sensitivity, specificity, and reliability. Within the context of quantifying posaconazole using high-performance liquid chromatography with diode array detection (HPLC-DAD) versus ultra-high-performance liquid chromatography with UV detection (UHPLC-UV), the wavelength range of 260-262 nm emerges as particularly significant. This application note explores the scientific rationale for this wavelength selection and provides detailed protocols for its implementation in pharmaceutical quality control and bioanalytical applications, supported by experimental data from current research.
The fundamental principle underlying UV detection in liquid chromatography is the Beer-Lambert Law, which establishes that absorbance (A) is proportional to the concentration (c) of the analyte in solution, the pathlength (b) of the flow cell, and the molar absorptivity (ε) of the compound at a specific wavelength [17] [18]. Mathematically, this is expressed as A = εbc. A detector measures the absorbance of ultraviolet light by sample components as they elute from the chromatography column, converting this signal into peaks whose areas can be correlated to analyte concentration [19]. The maximum absorbance wavelength (λmax) represents the characteristic wavelength of the absorption peak in the UV spectrum of a chromophoric molecule and is often selected as the monitoring wavelength in HPLC for optimal sensitivity and peak identification [18].
Theoretical Foundations of Wavelength Selection
Electronic Transitions and Chromophores
The absorption of UV radiation by organic molecules occurs when electrons transition from ground state to excited state energy levels. Posaconazole, with its extended conjugated system and aromatic triazole rings, contains chromophores—structural moieties that absorb UV or visible light [18]. The specific molecular structure of posaconazole, characterized by a difluorophenyl tetrahydrofuran group linked to a triazolinone ring system through a piperazinyl phenyl bridge, creates a complex chromophore with well-defined electronic transition properties [1]. These transitions result in absorption maxima in the UV region, particularly around 260-262 nm, where the molar absorptivity provides sufficient sensitivity for pharmaceutical analysis.
The Role of Solvent Systems
The choice of solvent and mobile phase composition significantly influences UV absorption characteristics. solvents must possess adequate transparency at the selected detection wavelength to avoid interference with analyte detection. For detection at 260-262 nm, common HPLC solvents such as methanol (cutoff ~210 nm), acetonitrile (cutoff ~195 nm), and aqueous buffers are highly suitable due to their low absorbance in this spectral region [20]. The organic modifier percentage in the mobile phase can cause minor shifts in absorption maxima, though research indicates posaconazole maintains strong absorbance at 262 nm across various mobile phase compositions [1] [21] [3].
Experimental Determination of Optimal Wavelength
Protocol for Wavelength Selection
Materials and Reagents:
- Posaconazole reference standard
- HPLC-grade methanol or acetonitrile
- Volumetric flasks (10 mL, 100 mL)
- UV-Vis spectrophotometer with quartz cuvettes
- Analytical balance
Procedure:
- Prepare a stock solution of posaconazole at approximately 100 μg/mL in methanol or acetonitrile.
- Dilute the stock solution to prepare a series of concentrations (e.g., 5, 10, 15, 20 μg/mL) using the same solvent.
- Fill a quartz cuvette with the blank solvent and record a baseline spectrum.
- For each diluted standard, measure the absorbance across the UV range of 200-400 nm.
- Identify the wavelength of maximum absorbance (λmax) from the resulting spectra.
- Confirm reproducibility across concentrations and on different days.
Expected Results: Multiple studies have consistently identified the maximum absorbance wavelength for posaconazole between 260 nm and 262 nm [1] [21] [3]. For instance, research conducted in 2024 confirmed strong posaconazole absorbance at 262 nm, making it ideal for HPLC-UV detection in plasma samples [3].
Rationale for 260-262 nm Selection
The selection of 262 nm for posaconazole analysis is not arbitrary but based on the compound's intrinsic absorption properties. This wavelength corresponds to electronic transitions within the conjugated system of posaconazole, particularly the π→π* transitions of its aromatic rings and triazole moieties [1]. The molar absorptivity at this wavelength provides excellent sensitivity for quantification, while minimizing potential interference from common excipients and mobile phase components that may absorb at lower wavelengths.
Table 1: Summary of Wavelength Selection in Recent Posaconazole HPLC Studies
| Study | Detection Wavelength | Matrix | Sensitivity Achieved |
|---|---|---|---|
| HPLC-DAD Method [1] | 262 nm | Bulk powder and suspension | LOD: 0.82 μg/mL; LOQ: 2.73 μg/mL |
| UHPLC-UV Method [1] | 262 nm | Bulk powder and suspension | LOD: 1.04 μg/mL; LOQ: 3.16 μg/mL |
| Reverse-Phase HPLC [21] | 262 nm | Bulk and marketed tablet | Linear range: 2-20 μg/mL |
| HPLC-UV for Plasma [3] | 262 nm | Rat plasma | LOQ: 50 ng/mL |
Comparative Analysis of HPLC-DAD vs. UHPLC-UV for Posaconazole Quantification
Method Parameters and Performance Characteristics
The detection wavelength of 262 nm has been successfully implemented across both conventional HPLC and UHPLC platforms for posaconazole quantification, with each approach offering distinct advantages.
Table 2: Comparative Chromatographic Conditions for Posaconazole Analysis at 262 nm
| Parameter | HPLC-DAD Method [1] | UHPLC-UV Method [1] | Recent HPLC Method [21] |
|---|---|---|---|
| Column | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) | Kinetex-C18 (2.1 × 50 mm, 1.3 μm) | Phenomenex Hyperclone C18 (250 × 4.6 mm, 5 μm) |
| Mobile Phase | Gradient: Acetonitrile:15 mM KH₂PO₄ (30:70 to 80:20) | Isocratic: Acetonitrile:15 mM KH₂PO₄ (45:55) | Isocratic: Acetonitrile:MeOH:10 mM phosphate buffer (pH 6.8) |
| Flow Rate | 1.5 mL/min | 0.4 mL/min | 0.8-1.2 mL/min |
| Run Time | 11 minutes | 3 minutes | Not specified |
| Injection Volume | 20-50 μL | 5 μL | 20 μL |
| Linearity | 5-50 μg/mL (r² > 0.999) | 5-50 μg/mL (r² > 0.999) | 2-20 μg/mL |
Detailed Experimental Protocols
Protocol for HPLC-DAD Analysis of Posaconazole in Suspension Dosage Form
Materials and Reagents:
- Posaconazole reference standard
- Itraconazole internal standard
- HPLC-grade acetonitrile and methanol
- Potassium dihydrogen orthophosphate (KH₂PO₄)
- High-purity distilled water
- Noxafil oral suspension (40 mg/mL) or equivalent
- Zorbax SB-C18 column (4.6 × 250 mm, 5 μm)
- HPLC system with DAD detector
Mobile Phase Preparation:
- Prepare 15 mM potassium dihydrogen orthophosphate solution by dissolving 2.04 g in 1L of HPLC-grade water.
- Filter the aqueous solution through a 0.45 μm membrane filter.
- Prepare the mobile phase as a gradient from acetonitrile:phosphate buffer (30:70) to (80:20) over 7 minutes.
- Degas the mobile phase by sonication for 10 minutes before use.
Standard Solution Preparation:
- Prepare a 100 μg/mL posaconazole stock solution in methanol.
- Prepare a 100 μg/mL itraconazole (internal standard) stock solution in methanol.
- Prepare calibration standards in the range of 5-50 μg/mL posaconazole with fixed concentration of internal standard (10 μg/mL).
Sample Preparation:
- Dilute 0.1 mL of posaconazole oral suspension (40 mg/mL) to 10 mL with methanol (S1).
- Add 10 μg/mL internal standard to 0.1 mL of S1 and dilute with methanol to a final volume of 1 mL (S2).
- Vortex mix for 10 seconds and centrifuge if necessary.
- Inject 20 μL into the HPLC system.
Chromatographic Conditions:
- Detection wavelength: 262 nm
- Flow rate: 1.5 mL/min
- Column temperature: 25°C
- Gradient elution: 30:70 to 80:20 acetonitrile:buffer over 7 minutes
- Total run time: 11 minutes
Protocol for UHPLC-UV Analysis of Posaconazole
Materials and Reagents:
- Kinetex-C18 column (2.1 × 50 mm, 1.3 μm)
- UHPLC system with UV detector
- All other reagents as in HPLC-DAD method
Mobile Phase Preparation:
- Prepare isocratic mobile phase of acetonitrile:15 mM KH₂PO₄ (45:55).
- Filter through 0.22 μm membrane filter and degas.
Sample Preparation:
- Follow the same procedure as for HPLC-DAD.
- Inject 5 μL into the UHPLC system.
Chromatographic Conditions:
- Detection wavelength: 262 nm
- Flow rate: 0.4 mL/min
- Column temperature: 40°C
- Isocratic elution
- Total run time: 3 minutes
Essential Research Reagent Solutions
The following table summarizes key reagents and materials required for implementing posaconazole quantification methods at 262 nm detection.
Table 3: Essential Research Reagents for Posaconazole HPLC Analysis
| Reagent/Material | Specification | Function in Analysis |
|---|---|---|
| Posaconazole Reference Standard | Pharmaceutical secondary standard | Primary standard for calibration curve preparation |
| Itraconazole | Internal standard grade | Internal standard for retention time normalization and quantification [1] |
| Acetonitrile | HPLC gradient grade | Organic modifier in mobile phase; solvent for standard and sample preparation |
| Methanol | HPLC grade | Solvent for stock solutions and sample extraction |
| Potassium Dihydrogen Orthophosphate | Analytical grade | Buffer component for mobile phase to control pH and improve peak shape |
| C18 Chromatographic Column | 5 μm for HPLC; 1.3 μm for UHPLC | Stationary phase for reverse-phase separation [1] |
| Water | HPLC grade (e.g., Milli-Q) | Aqueous component of mobile phase |
Method Validation Parameters
Methods employing 262 nm detection for posaconazole have demonstrated excellent validation characteristics according to International Conference on Harmonisation (ICH) guidelines [1] [21]:
- Linearity: Typically r² > 0.999 over concentration ranges from 2-50 μg/mL
- Precision: CV% < 3% for both intra-day and inter-day variations
- Accuracy: Percentage recovery generally between 99-101%
- Specificity: No interference from excipients or degradation products at 262 nm
- Sensitivity: Limits of detection as low as 0.82 μg/mL for HPLC and 50 ng/mL for advanced methods [1] [3]
Detection Workflow and Wavelength Selection Rationale
The following diagram illustrates the logical workflow for wavelength selection and implementation in posaconazole analysis:
Wavelength Selection Workflow
The selection of 260-262 nm as the detection wavelength for posaconazole analysis represents a scientifically grounded choice based on the compound's intrinsic absorption properties. This wavelength provides optimal sensitivity and specificity for quantification across various matrices including bulk drug substance, pharmaceutical formulations, and biological samples. The consistent application of this wavelength in both HPLC-DAD and UHPLC-UV methods demonstrates its robustness and reliability for pharmaceutical quality control and bioanalytical applications. Researchers can successfully implement the detailed protocols provided in this application note to develop validated analytical methods for posaconazole quantification, ensuring accurate and reproducible results in both conventional and ultra-high-performance liquid chromatography platforms.
The accurate quantification of Posaconazole (PSZ), a broad-spectrum triazole antifungal agent, is a critical requirement in pharmaceutical quality control and therapeutic drug monitoring [1] [2]. Its applications span from the analysis of raw materials and formulated products like bulk powders, oral suspensions, and tablets, to the determination of concentration levels in biological fluids such as plasma for pharmacokinetic studies [1] [3] [4]. The selection of an appropriate analytical technique is paramount for achieving reliable results. High-Performance Liquid Chromatography with a Diode Array Detector (HPLC-DAD) and Ultra-High-Performance Liquid Chromatography with an Ultraviolet detector (UHPLC-UV) are two pivotal techniques employed for this purpose [1]. This document provides detailed application notes and protocols for the quantitation of Posaconazole across this spectrum, framing the discussion within a broader thesis comparing HPLC-DAD and UHPLC-UV methodologies.
Comparative Analytical Techniques: HPLC-DAD vs. UHPLC-UV
The core of the methodological choice often hinges on the balance between analytical performance and practical laboratory constraints. HPLC-DAD is a well-established, robust, and widely available workhorse in quality control laboratories. In contrast, UHPLC-UV leverages columns packed with smaller particles (<2 µm) and operates at higher pressures to deliver superior chromatographic separation, speed, and solvent economy [1]. A direct comparison of their performance in quantifying Posaconazole is summarized in Table 1.
Table 1: Quantitative Performance Comparison of HPLC-DAD and UHPLC-UV Methods for Posaconazole Assay
| Parameter | HPLC-DAD Method [1] | UHPLC-UV Method [1] | HPLC-UV for Plasma [3] |
|---|---|---|---|
| Linear Range | 5–50 µg/mL | 5–50 µg/mL | 50–2000 ng/mL |
| Correlation Coefficient (r²) | >0.999 | >0.999 | Not Specified |
| Limit of Detection (LOD) | 0.82 µg/mL | 1.04 µg/mL | Not Specified |
| Limit of Quantitation (LOQ) | 2.73 µg/mL | 3.16 µg/mL | 50 ng/mL |
| Run Time | 11 minutes | 3 minutes | 8.2 minutes |
| Precision (CV%) | <3% | <3% | Validated per ICH |
| Key Advantages | Robust, widely available | Faster analysis, less solvent consumption, superior separation | Low plasma volume requirement (100 µL), high recovery (>98%) |
The following diagram illustrates the decision-making workflow for selecting the appropriate analytical technique and method based on the sample type and analytical requirements.
Detailed Experimental Protocols
Protocol 1: Quantitation in Bulk Powder and Oral Suspension using HPLC-DAD and UHPLC-UV
This protocol is adapted from a direct comparative study and is ideal for quality control of pharmaceutical formulations [1].
Materials and Reagents
- Posaconazole Bulk Powder and Noxafil Oral Suspension (40 mg/mL).
- HPLC-grade solvents: Methanol and Acetonitrile.
- Water, high purity distilled.
- Buffer Salt: Analytical grade Potassium Dihydrogen Orthophosphate (KH₂PO₄).
- Internal Standard (IS): Itraconazole.
Instrumentation and Chromatographic Conditions
Table 2: Chromatographic Conditions for Formulation Analysis
| Condition | HPLC-DAD Method | UHPLC-UV Method |
|---|---|---|
| Column | Zorbax SB-C18 (4.6 × 250 mm, 5 µm) | Kinetex-C18 (2.1 × 50 mm, 1.3 µm) |
| Mobile Phase | Gradient: Acetonitrile : 15 mM KH₂PO₄ (30:70 to 80:20 over 7 min) | Isocratic: Acetonitrile : 15 mM KH₂PO₄ (45:55) |
| Flow Rate | 1.5 mL/min | 0.4 mL/min |
| Detection Wavelength | 262 nm | 262 nm |
| Injection Volume | 20-50 µL | 5 µL |
| Column Temperature | 25°C | 40°C |
| Run Time | 11 min | 3 min |
Sample Preparation
- Standard Stock Solution (100 µg/mL): Accurately weigh 10 mg of Posaconazole reference standard and dissolve in 100 mL of methanol.
- Calibration Standards: Prepare working solutions from the stock by dilution with methanol to concentrations ranging from 5–50 µg/mL. Add a fixed concentration of Itraconazole (e.g., 10 µg/mL) as the Internal Standard.
- Oral Suspension Sample:
- Dilute 0.1 mL of the suspension to 10 mL with methanol (Solution S1).
- Further dilute 0.1 mL of S1 with methanol to a final volume of 1 mL, containing the same concentration of Internal Standard as the calibration standards (Solution S2).
- Vortex mix thoroughly for 10 seconds before injection.
Analysis
- Inject the calibration standards and the prepared sample solutions in replicates (n=4).
- Construct a calibration curve by plotting the peak area ratio of Posaconazole to the Internal Standard against the nominal concentration.
- Calculate the concentration in the unknown sample using the regression equation from the calibration curve.
Protocol 2: Quantitation in Low-Volume Plasma using HPLC-UV
This protocol is designed for preclinical pharmacokinetic studies where sample volume is limited, such as in rat studies [3].
Materials and Reagents
- Posaconazole Standard.
- Internal Standard: Diazepam.
- HPLC-grade solvents: Acetonitrile, Methanol, and Tertiary Butyl Methyl Ether (TBME).
- Salts and Reagents: Sodium Chloride (NaCl), Sodium Hydroxide (NaOH), Orthophosphoric Acid.
Instrumentation and Chromatographic Conditions
- Column: PerfectSil Target C8 (250 × 4.6 mm, 5 µm).
- Mobile Phase: Combination of ACN and MeOH (total organic phase 58%) with phosphate buffer (pH 7). The ratio of MeOH in the organic phase is 6%.
- Flow Rate: 1.2 mL/min.
- Detection Wavelength: 262 nm.
- Column Temperature: 39°C.
- Retention Time: ~8.2 minutes for Posaconazole.
Sample Preparation (Liquid-Liquid Extraction)
- Plasma Sample: Aliquot 100 µL of plasma into a tube.
- Add Internal Standard: Add a known amount of Diazepam IS solution.
- Alkalization: Add 10% w/v NaCl solution and adjust the plasma pH to 11 using NaOH to enhance the extraction efficiency of the drug.
- Extraction: Add 500 µL of TBME extraction solvent. Vortex mix for 10 minutes.
- Centrifugation: Centrifuge at high speed (e.g., 10,000 ×g) for 1 minute to separate the phases.
- Collection: Transfer the organic (upper) layer to a new tube.
- Evaporation: Evaporate the organic solvent to dryness under a gentle stream of nitrogen or in a vacuum concentrator.
- Reconstitution: Reconstitute the dry residue with 100-200 µL of the HPLC mobile phase. Vortex thoroughly and inject into the HPLC system.
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful analysis requires high-quality materials and a clear understanding of their function. Table 3 lists key reagents and their roles in the quantification of Posaconazole.
Table 3: Key Research Reagent Solutions for Posaconazole Quantitation
| Reagent/Material | Function/Explanation |
|---|---|
| C18 Chromatography Columns | The most common stationary phase for reverse-phase separation of Posaconazole. Particle size (5µm vs. sub-2µm) dictates HPLC vs. UHPLC application [1] [2]. |
| Acetonitrile & Methanol (HPLC Grade) | Organic modifiers in the mobile phase; they compete with the analyte for the stationary phase, controlling retention time and separation efficiency [1] [3]. |
| Potassium Dihydrogen Phosphate Buffer | Aqueous component of the mobile phase; buffers the pH to ensure consistent ionization and reproducible retention times [1] [21]. |
| Itraconazole / Diazepam (IS) | Internal Standard used to correct for variability in sample preparation, injection volume, and instrument performance [1] [22]. |
| Tertiary Butyl Methyl Ether (TBME) | A solvent for liquid-liquid extraction; effectively precipitates proteins and extracts Posaconazole from plasma with high recovery (>98%) [3]. |
The quantitative analysis of Posaconazole across its application spectrum—from pharmaceutical formulations to complex biological matrices—is reliably supported by both HPLC-DAD and UHPLC-UV techniques. The choice between them depends on the specific analytical demands: UHPLC-UV offers superior speed and efficiency for high-throughput formulation analysis, while robust and optimized HPLC methods remain indispensable for challenging tasks like quantifying drugs in low-volume plasma samples for preclinical pharmacokinetic studies. The protocols and data presented herein provide a validated foundation for researchers to implement these methods, ensuring accurate and precise quantitation of Posaconazole in diverse settings.
Step-by-Step Method Development for Posaconazole in Formulations and Plasma
Within the framework of research comparing High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) and Ultra-High Performance Liquid Chromatography with Ultraviolet Detection (UHPLC-UV) for the quantitation of posaconazole, this application note provides detailed methodological protocols. Posaconazole is a broad-spectrum triazole antifungal agent, and its accurate quantification is crucial for quality control of pharmaceutical products and pharmacokinetic studies [1] [23] [2]. Although numerous methods exist for its analysis in biological fluids, well-characterized protocols for finished products are less commonly reported, and official pharmacopoeial methods are absent [1] [23]. This document outlines the development and validation of two distinct, stability-indicating chromatographic methods, summarizing key parameters into structured tables and providing step-by-step experimental procedures to facilitate replication by researchers and pharmaceutical analysts.
Comparative Chromatographic Conditions and Method Validation
The development of a robust HPLC method requires careful optimization of the stationary phase, mobile phase composition, and gradient profile. Below, a comparative summary of the established methods for posaconazole is provided, followed by a detailed protocol for the HPLC-DAD method.
Table 1: Comparative Chromatographic Conditions for Posaconazole Quantitation
| Parameter | HPLC-DAD Method [1] | UHPLC-UV Method [1] | Stability-Indicating HPLC-UV Method [23] |
|---|---|---|---|
| Column | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) | Kinetex-C18 (2.1 × 50 mm, 1.3 μm) | C8 Column (Specific type not stated) |
| Mobile Phase | Acetonitrile : 15 mM KH₂PO₄ (Gradient: 30:70 to 80:20 over 7 min) | Acetonitrile : 15 mM KH₂PO₄ (45:55, Isocratic) | Methanol : Water (75:25, v/v) |
| Flow Rate | 1.5 mL/min | 0.4 mL/min | 1.0 mL/min |
| Injection Volume | 20-50 μL | 5 μL | 20 μL |
| Detection Wavelength | 262 nm | 262 nm | 260 nm |
| Column Temperature | 25°C | 40°C | Ambient |
| Run Time | 11 minutes | 3 minutes | ~8.5 minutes |
| Internal Standard | Itraconazole | Itraconazole | Not Specified |
Table 2: Summary of Method Validation Data
| Validation Parameter | HPLC-DAD Method [1] | Stability-Indicating HPLC-UV Method [23] | New HPLC-UV Method (2023) [2] |
|---|---|---|---|
| Linearity Range | 5–50 μg/mL | 5–60 μg/mL | 2–20 μg/mL |
| Correlation Coefficient (r²) | > 0.999 | 0.9996 | Not Specified (Reported as linear) |
| Precision (RSD%) | < 3% | Intra-day: 0.86-1.22%Inter-day: 1.21% | Intra-day & Inter-day: < 1% |
| Accuracy (% Recovery) | < 3% Error | Mean Recovery: 98.13% | Bulk: 99.01%Dosage Form: 99.05% |
| Limit of Detection (LOD) | 0.82 μg/mL | Not Specified | Calculated via formula |
| Limit of Quantitation (LOQ) | 2.73 μg/mL | Not Specified | Calculated via formula |
Experimental Protocols
3.1.1 Materials and Reagents
- Posaconazole Reference Standard: Selleckchem (Houston, TX, USA).
- Itraconazole (Internal Standard): Obtain from pharmaceutical source (e.g., Nifty Labs PVT Ltd.).
- Solvents: HPLC grade methanol and acetonitrile (Fisher Scientific).
- Water: High purity distilled water.
- Buffer Salt: Analytical grade potassium dihydrogen orthophosphate (KH₂PO₄).
- Dosage Form: Noxafil 40 mg/mL oral suspension (Patheon Inc.).
3.1.2 Instrumentation and Chromatographic Conditions
- HPLC System: Agilent 1200 series, equipped with a quaternary pump, vacuum degasser, diode array detector (DAD), and autosampler.
- Data Software: Agilent ChemStation.
- Column: Zorbax SB-C18 (4.6 × 250 mm, 5 μm).
- Mobile Phase: Acetonitrile (A) and 15 mM potassium dihydrogen orthophosphate (B). Use a gradient elution: start at 30:70 (A:B), linear gradient to 80:20 over 7 minutes.
- Flow Rate: 1.5 mL/min.
- Detection: DAD, 262 nm.
- Injection Volume: 20 μL.
- Column Temperature: 25°C.
3.1.3 Preparation of Standard Solutions
- Stock Solution of PSZ (100 μg/mL): Accurately weigh 10 mg of posaconazole reference standard and dissolve in 100 mL of methanol.
- Stock Solution of IS (100 μg/mL): Accurately weigh 10 mg of itraconazole and dissolve in 100 mL of methanol.
- Calibration Standards: Prepare working solutions from the stock solution by serial dilution with methanol. Prepare concentrations ranging from 5 to 50 μg/mL of posaconazole. To each calibration standard, add itraconazole IS to a final concentration of 10 μg/mL. Make up the final volume to 1 mL with methanol. Vortex mix for 10 seconds.
3.1.4 Sample Preparation from Oral Suspension
- Pipette 0.1 mL of the posaconazole oral suspension (40 mg/mL) into a 10 mL volumetric flask. Dilute to volume with methanol and mix well (Solution S1).
- Transfer 0.1 mL of S1 supernatant into a 2.5 mL polypropylene microcentrifuge tube.
- Add 10 μg/mL of itraconazole IS solution.
- Dilute to a final volume of 1 mL with methanol (Solution S2).
- Vortex mix thoroughly and inject into the HPLC system.
3.1.5 System Suitability and Validation
- Perform validation in accordance with ICH guidelines [1] [24].
- Assess system suitability by injecting six replicates of a standard at the middle concentration of the calibration range. The relative standard deviation (RSD%) of the peak area and retention time for posaconazole should be less than 2%.
- Validate the method for specificity, linearity, precision (intra-day and inter-day), accuracy (recovery), LOD, and LOQ as summarized in Table 2.
A stability-indicating method must demonstrate that the analyte peak is free from interference from degradation products.
3.2.1 Stress Conditions
- Acidic Degradation: Expose posaconazole solution to 0.1 M HCl at room temperature for 10 days.
- Basic Degradation: Expose posaconazole solution to 0.1 M NaOH at room temperature for 10 days.
- Oxidative Degradation: Expose posaconazole solution to 3% H₂O₂ at room temperature for 10 days.
- Thermal Degradation: Expose the solid drug to dry heat (e.g., 60°C) for 10 days.
3.2.2 Analysis
- After the stress period, neutralize the acid/base samples and prepare all samples in the mobile phase at an appropriate concentration.
- Inject the samples into the HPLC system using the developed method.
- Apply peak purity tool (available with DAD) to the main posaconazole peak to ensure it is pure and free from co-eluting degradation products. The method is specific if the peak purity index is above a defined threshold (e.g., 990) and there is no interference at the retention time of posaconazole.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials and Reagents for HPLC-DAD Analysis of Posaconazole
| Item | Function / Purpose | Examples / Specifications |
|---|---|---|
| C18 Reverse-Phase Column | Stationary phase for chromatographic separation. | Zorbax SB-C18 (250 x 4.6 mm, 5 µm) [1]; Phenomenex Hyperclone C18 [2]. |
| Acetonitrile (HPLC Grade) | Organic modifier in the mobile phase. | Provides efficient elution and sharp peak shape. Fisher Scientific [1]. |
| Methanol (HPLC Grade) | Solvent for stock solutions & mobile phase component. | Used for sample dissolution and dilution [23]. |
| Buffer Salts | Aqueous component of mobile phase to control pH. | Potassium dihydrogen phosphate (KH₂PO₄) [1]; Ammonium acetate [25]. |
| Posaconazole Reference Standard | Primary standard for calibration curve. | Enables accurate quantification of the drug. Selleckchem [1]. |
| Internal Standard | Corrects for analytical variability. | Itraconazole [1] or Ketoconazole [25]. |
| Diode Array Detector (DAD) | Detection and peak purity assessment. | Agilent 1200/1290 series DAD; enables multi-wavelength detection and peak purity analysis [1] [26]. |
Discussion and Concluding Remarks
The detailed protocols and conditions provided herein enable the reliable quantification of posaconazole in bulk and pharmaceutical dosage forms using HPLC-DAD. The developed method is specific, as confirmed by forced degradation studies, which show no interference from degradation products, confirming its stability-indicating nature [23]. The method validation parameters, including linearity, precision, and accuracy, comply with ICH guidelines, making it suitable for quality control purposes [1] [24].
When positioned within a broader thesis comparing HPLC-DAD and UHPLC-UV, the UHPLC method demonstrates clear advantages in speed, solvent consumption, and chromatographic efficiency [1] [27]. The run time for UHPLC is significantly shorter (3 minutes vs. 11 minutes for HPLC), and solvent consumption is reduced four-fold, aligning with the principles of green chemistry [1] [27]. However, the choice between the two techniques depends on the available instrumentation and the specific requirements of the laboratory. The HPLC-DAD method remains a highly accessible, cost-effective, and robust solution for the routine analysis of posaconazole, providing a solid foundation for comparative studies.
Ultra-High Performance Liquid Chromatography with Ultraviolet detection (UHPLC-UV) represents a significant advancement in liquid chromatography, providing superior speed, resolution, and sensitivity compared to traditional High-Performance Liquid Chromatography (HPLC). This application note details optimized UHPLC-protocols for the quantification of posaconazole, a broad-spectrum triazole antifungal agent, framed within research comparing HPLC-Diode Array Detection (DAD) and UHPLC-UV methodologies [1]. The transition to UHPLC technology utilizing sub-2μm particles and operating at high pressures enables substantial reductions in analysis time and solvent consumption while maintaining or improving chromatographic performance [28] [29] [30]. These protocols are designed for researchers, scientists, and drug development professionals requiring robust, efficient analytical methods for antifungal drug analysis.
Comparative Method Summaries: HPLC-DAD vs. UHPLC-UV for Posaconazole
Table 1: Key Parameter Comparison for Posaconazole Analysis
| Parameter | HPLC-DAD Method [1] | UHPLC-UV Method [1] | Advanced UHPLC-UV Protocol |
|---|---|---|---|
| Column Dimensions | 4.6 × 250 mm | 2.1 × 50 mm | 2.1 × 100 mm |
| Particle Size | 5 μm (fully porous) | 1.3 μm (core-shell) | 1.7 μm (fully porous) |
| Mobile Phase | Gradient: ACN:15 mM KH₂PO₄ (30:70 to 80:20) | Isocratic: ACN:15 mM KH₂PO₄ (45:55) | Isocratic or Gradient optimized |
| Flow Rate | 1.5 mL/min | 0.4 mL/min | 0.4-0.6 mL/min |
| Analysis Time | 11 minutes | 3 minutes | <5 minutes |
| Injection Volume | 20-50 μL | 5 μL | 1-5 μL |
| Acetonitrile Consumption per Run | ~12 mL | ~1.2 mL | ~0.8-1.8 mL |
| Detection Wavelength | 262 nm | 262 nm | 262 nm |
| System Pressure | ~400 bar | ~1000 bar | 600-1500 bar |
Detailed UHPLC-UV Experimental Protocol for Posaconazole Quantification
Materials and Reagents
- Analytical Standards: Posaconazole (BrightGene Co., Ltd.); Internal Standard (e.g., Itraconazole or Diazepam)
- Mobile Phase Components: Acetonitrile (HPLC grade), Methanol (HPLC grade), Potassium Dihydrogen Orthophosphate (KH₂PO₄, analytical grade)
- Water: Deionized water, 18.2 MΩ·cm resistivity, filtered through 0.22 μm membrane
- Solvents for Sample Preparation: Methanol, Acetonitrile, Tertiary Butyl Methyl Ether (TBME)
Instrumentation and Column Configuration
- UHPLC System: Compatible with pressures up to 1500 bar (e.g., Agilent 1290 Infinity, Waters Acquity UPLC, Thermo Scientific Dionex)
- Column: Acquity BEH C18 (100 mm × 2.1 mm, 1.7 μm) or equivalent sub-2μm particle column [31] [30]
- Detection: UV detector set at 262 nm
- Data Acquisition: Software capable of high-speed data collection (≥20 Hz)
Mobile Phase Preparation
- Isocratic Mode: Prepare mixture of Acetonitrile and 15 mM Potassium Dihydrogen Orthophosphate (45:55, v/v)
- Buffer Preparation: Dissolve 2.04 g KH₂PO₄ in 1000 mL deionized water, adjust pH if necessary, filter through 0.22 μm membrane
- Mixing: Combine appropriate volumes of acetonitrile and buffer, degas by sonication or sparging with inert gas
Sample Preparation Procedure
- Standard Solutions: Prepare stock solution of posaconazole (1 mg/mL) in methanol
- Calibration Standards: Dilute stock solution with mobile phase or appropriate solvent to create calibration curve (e.g., 50-2000 ng/mL)
- Internal Standard: Add appropriate internal standard (e.g., 100 μL of 10 μg/mL solution) to all samples and standards
- Extraction: For plasma samples, employ liquid-liquid extraction with TBME or protein precipitation with acetonitrile (1:3 sample:solvent ratio)
- Centrifugation: Centrifuge at 14,000 × g for 10 minutes
- Reconstitution: Reconstitute dried extract in 100-200 μL of mobile phase
- Filtration: Filter through 0.22 μm membrane prior to injection
System Equilibration and Chromatographic Conditions
- Column Temperature: 40°C
- Flow Rate: 0.4 mL/min
- Injection Volume: 1-5 μL (using partial loop or needle overfill mode)
- Equilibration: Flush system with mobile phase for 10-15 column volumes until stable baseline achieved
- System Backpressure: Approximately 800-1200 bar under initial conditions
Method Validation Parameters
- Linearity: Evaluate over concentration range of 50-2000 ng/mL (r² > 0.999)
- Precision: Intra-day and inter-day precision (%RSD < 5%)
- Accuracy: 95-105% recovery
- Limit of Quantification (LOQ): ≤50 ng/mL [3]
- Specificity: No interference from sample matrix at retention time of analyte
UHPLC Method Development Workflow
UHPLC System Optimization Strategy
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Research Reagent Solutions for UHPLC-UV Analysis of Posaconazole
| Item | Function/Application | Examples/Specifications |
|---|---|---|
| Sub-2μm UHPLC Columns | High-efficiency separation core component; enables fast analysis with high resolution | Acquity BEH C18 (1.7μm), Kinetex C18 (1.3μm core-shell) [29] [1] |
| Matching Guard Columns | Protects analytical column from matrix components; extends column lifetime | Manufacturer-specific guard cartridges with identical stationary phase |
| HPLC-Grade Solvents | Mobile phase preparation; ensures minimal UV background and system compatibility | Low UV-absorbing acetonitrile and methanol [3] [1] |
| Buffer Salts | Mobile phase modifier; controls pH and improves separation | Potassium dihydrogen phosphate, ammonium acetate, ammonium formate (HPLC grade) [1] |
| Sample Preparation | Extraction and cleanup of posaconazole from biological matrices | TBME for liquid-liquid extraction; acetonitrile for protein precipitation [3] |
| Reference Standards | Method calibration and quality control | Certified posaconazole standard; appropriate internal standard [3] [1] |
| UHPLC System | High-pressure capable instrumentation; enables sub-2μm particle technology benefits | Systems rated for 1000-1500 bar pressure [28] [30] |
| Vial Inserts | Accommods low injection volumes; minimizes sample waste | 100-200μL inserts with low volume vials |
The implementation of UHPLC-UV methods utilizing sub-2μm columns provides significant advantages for posaconazole quantification compared to conventional HPLC-DAD approaches. The detailed protocols presented enable a 70-80% reduction in analysis time (from 11 to 3 minutes) and substantially reduce solvent consumption (from ~12 mL to ~1.2 mL per run) while maintaining analytical performance [1]. The optimized UHPLC conditions leverage high-pressure capabilities (600-1500 bar), isocratic or shallow gradient elution, and minimized system dispersion to achieve rapid, reproducible results ideal for high-throughput environments. These application notes provide researchers and pharmaceutical scientists with comprehensive methodologies to implement robust UHPLC-UV analysis of posaconazole, contributing valuable approaches to the broader comparison of HPLC versus UHPLC technologies in antifungal drug development and therapeutic monitoring.
Within the broader scope of a thesis investigating the quantitation of posaconazole (PCZ) using High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) versus Ultra-High-Performance Liquid Chromatography with Ultraviolet Detection (UHPLC-UV), sample preparation stands as a critical foundational step. The choice of preparation technique directly impacts the sensitivity, specificity, and overall reliability of the chromatographic analysis. For the accurate quantification of PCZ in different sample matrices, two distinct sample preparation strategies are paramount: direct dilution for formulated products and liquid-liquid extraction (LLE) for biological samples like plasma. This document provides detailed application notes and protocols for these essential techniques, framing them within the context of advanced chromatographic method development and validation.
Experimental Protocols
Direct Dilution for Formulation Analysis
The direct dilution method is a straightforward and efficient technique suitable for the analysis of PCZ in pharmaceutical formulations, such as oral suspensions. This protocol is optimized to minimize excipient interference and is compatible with both HPLC-DAD and UHPLC-UV analyses [1].
Materials and Reagents
- Posaconazole oral suspension (e.g., Noxafil 40 mg/mL)
- HPLC-grade methanol
- Volumetric flasks (10 mL, 100 mL)
- Microcentrifuge tubes (2.5 mL)
- Analytical balance
Step-by-Step Procedure
- Stock Solution Preparation: Precisely pipette 0.1 mL of the PCZ oral suspension into a 10 mL volumetric flask. Dilute to the mark with methanol to obtain a nominal concentration of 400 µg/mL (Solution S1).
- Working Solution Preparation: Transfer 0.1 mL of Solution S1 into a 2.5 mL polypropylene microcentrifuge tube.
- Internal Standard Addition: Add a suitable volume of internal standard (e.g., itraconazole) solution to achieve a final concentration of 10 µg/mL.
- Final Dilution: Dilute the mixture to a final volume of 1 mL with methanol and vortex-mix at high speed for 10 seconds.
- Chromatographic Analysis: Centrifuge if necessary and inject the supernatant into the HPLC or UHPLC system. The typical injection volume is 20 µL for HPLC and 5 µL for UHPLC [1].
Liquid-Liquid Extraction for Plasma Samples
LLE is preferred for plasma samples to remove proteins and endogenous interfering compounds, thereby enhancing assay selectivity and protecting the chromatographic column. This protocol is validated for the extraction of PCZ from low-volume rat plasma, crucial for preclinical pharmacokinetic studies [4] [3].
Materials and Reagents
- Plasma samples (rat or human)
- Posaconazole and Internal Standard (e.g., Itraconazole or Diazepam) stock solutions
- Diethyl ether (HPLC grade) or Tertiary Butyl Methyl Ether (TBME)
- Sodium hydroxide (NaOH) solution (0.1 M or 1 M)
- Sodium chloride (NaCl)
- Vortex mixer
- Centrifuge
- Glass test tubes
- Nitrogen evaporator
Optimized Extraction Procedure
- Plasma Aliquot: Pipette 100-200 µL of plasma into a glass test tube [4] [3].
- Internal Standard Addition: Add the appropriate volume of internal standard working solution.
- Basification: Add 30 µL of 0.1 M NaOH or adjust plasma pH to ~11 using 1 M NaOH to ensure the analyte is in its un-ionized form, facilitating partition into the organic solvent [4] [3].
- Salt Addition (Optional): Add 10% w/v NaCl to improve the recovery of the analyte by reducing its solubility in the aqueous phase [3].
- Liquid-Liquid Extraction: Add 0.5 - 6 mL of diethyl ether (the volume can be optimized based on recovery). Cap the tube and vortex-mix vigorously for 2-10 minutes. The optimal condition was found to be 10 minutes of extraction with 500 µL of TBME [4] [3].
- Centrifugation: Centrifuge the mixture at approximately 2500-5000 ×g for 5-10 minutes to achieve clean phase separation [4] [32].
- Organic Layer Transfer: Carefully transfer the upper organic layer to a new clean glass tube.
- Evaporation: Evaporate the organic solvent to dryness under a gentle stream of nitrogen in a 37-40°C water bath [32] [3].
- Reconstitution: Reconstitute the dry residue with 100-250 µL of methanol or the mobile phase. Vortex thoroughly to ensure complete dissolution.
- Analysis: Inject an aliquot (50-80 µL for HPLC, lower for UHPLC) into the chromatographic system [4] [1].
Table 1: Optimized Conditions for Liquid-Liquid Extraction of Posaconazole from Plasma
| Parameter | Recommended Condition | Variations/Notes |
|---|---|---|
| Plasma Volume | 100 - 200 µL | Suitable for low-volume samples from small animals [3]. |
| Basification | 0.1 M NaOH or pH 11 | Converts PCZ to neutral form for better extraction efficiency [4] [3]. |
| Extraction Solvent | Diethyl Ether, TBME | TBME showed high recovery (>98%) with low volume [3]. |
| Extraction Volume | 0.5 - 6 mL | Lower volumes (0.5 mL) are economically and environmentally friendly [4] [3]. |
| Extraction Time | 10 minutes | Optimized for maximum recovery [3]. |
| Centrifuge Time | 1 - 10 minutes | 1 minute was sufficient at optimized conditions [3]. |
Comparative Analysis of Techniques in HPLC-DAD vs. UHPLC-UV Context
The choice of sample preparation is intrinsically linked to the chromatographic platform. The following table summarizes key methodological and performance characteristics when these sample preparation techniques are applied in the context of a thesis comparing HPLC-DAD and UHPLC-UV.
Table 2: Comparison of Sample Preparation and Analytical Techniques for Posaconazole Quantitation
| Aspect | Direct Dilution (Formulations) | Liquid-Liquid Extraction (Plasma) |
|---|---|---|
| Primary Goal | Simple dissolution, excipient dilution [1] | Protein removal, analyte purification, and concentration [4] [3] |
| Typical Matrix | Oral suspension, tablet formulation [1] [2] | Rat/Human plasma or serum [4] [25] |
| HPLC-DAD Analysis | Column: Zorbax SB-C18 (4.6 × 250 mm, 5 µm). Run time: ~11 min [1] | Column: HC-C18 (4.6 × 250 mm, 5 µm). Run time: ~11 min [4] |
| UHPLC-UV Analysis | Column: Kinetex-C18 (2.1 × 50 mm, 1.3 µm). Run time: ~3 min [1] | Column: PerfectSil Target C8 (250 × 4.6 mm, 5 µm). Run time: ~8.2 min [3] |
| Linear Range | 5–50 µg/mL (for formulation assay) [1] | 50–5000 ng/mL (50–2000 ng/mL in plasma) [4] [3] |
| Limit of Quantification (LOQ) | 2.73 µg/mL (HPLC-DAD) [1] | 50 ng/mL in plasma [4] [3] |
| Key Advantage | Rapid, minimal steps, high recovery (>99%) [2] | Cleaner chromatograms, higher sensitivity, removes matrix effects [4] [3] |
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagent Solutions and Materials
| Item | Function/Application | Example & Notes |
|---|---|---|
| Internal Standard (IS) | Normalizes analytical variability during sample preparation and injection. | Itraconazole: Used in formulation and plasma analysis with HPLC-DAD [4] [1]. Diazepam: Used as IS in plasma analysis with HPLC-UV [3]. |
| Extraction Solvents | Medium for extracting analytes from the aqueous plasma matrix. | Diethyl Ether: Common, effective solvent for LLE [4] [32]. TBME: Demonstrated >98% recovery for PCZ with low volume [3]. |
| Buffers & pH Adjusters | Adjust pH to control the ionization state of the analyte for efficient extraction. | 0.1 M NaOH: Used to basify plasma samples (pH ~11) for LLE [4] [3]. Phosphate Buffers: Component of mobile phase (e.g., 15 mM KH₂PO₄) [4] [1]. |
| Chromatography Columns | Stationary phase for analytical separation. | HPLC (C18): Zorbax SB-C18, 4.6 × 250 mm, 5 µm [4] [1]. UHPLC (C18): Kinetex-C18, 2.1 × 50 mm, 1.3 µm [1]. |
| Protein Precipitants | Alternative to LLE for plasma; precipitates proteins. | Methanol: Used in a simple dilution/precipitation method for serum/plasma, followed by direct injection of the supernatant [25]. |
Workflow Visualization
Sample Preparation Workflow for HPLC/UHPLC Analysis
HPLC-DAD vs. UHPLC-UV Chromatographic Conditions
In the development of robust bioanalytical methods for monitoring antifungal drugs such as posaconazole, the selection of an appropriate internal standard (IS) is a critical factor for ensuring accuracy and precision. This application note details the strategic use of itraconazole as a reliable internal standard for the quantitation of posaconazole in biological matrices. The content is framed within a comprehensive research project comparing High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) and Ultra-High-Performance Liquid Chromatography with Ultraviolet Detection (UHPLC-UV) platforms. Itraconazole presents an ideal IS candidate due to its structural similarity to posaconazole, comparable chemical properties, and well-understood chromatographic behavior, which collectively facilitate precise correction for variability in sample preparation and analysis [1] [33]. This protocol provides detailed methodologies for implementing itraconazole as an IS, complete with validation data and application workflows tailored for researchers and drug development professionals.
Research Reagent Solutions
The following table catalogues the essential materials and reagents required for the successful implementation of this analytical method.
Table 1: Essential Research Reagents and Materials
| Item | Function | Specific Example / Note |
|---|---|---|
| Itraconazole (Standard) | Serves as the Internal Standard (IS) | Structural analogue of posaconazole; corrects for procedural losses [1]. |
| Posaconazole (Analyte) | Target compound for quantification | Model poorly soluble antifungal drug [1]. |
| HPLC-Grade Methanol & Acetonitrile | Solvent for stock solutions, protein precipitation, and mobile phase | Ensures minimal background interference [34] [33]. |
| Ammonium Formate/Acetate | Mobile phase additive | Improves chromatographic peak shape and MS compatibility [34] [35]. |
| Acid Modifiers (e.g., Formic Acid) | Mobile phase additive | Enhances ionization in MS and controls silica surface chemistry [34] [27]. |
| C18 Chromatographic Columns | Stationary phase for analytical separation | HPLC: e.g., Zorbax SB-C18 (4.6 × 250 mm, 5 μm). UHPLC: e.g., Kinetex-C18 (2.1 × 50 mm, 1.3 μm) [1]. |
| Drug-Free Human Plasma/Serum | Biological matrix for calibration standards and quality controls | Sourced from certified vendors; used for preparing calibration curves [34] [25]. |
Experimental Protocols
Protocol 1: Sample Preparation via Protein Precipitation
This optimized protocol ensures efficient recovery of both posaconazole and the itraconazole internal standard while effectively removing plasma proteins [34] [33] [25].
Workflow Diagram: Sample Preparation
Materials:
- Itraconazole stock solution (100 µg/mL in methanol)
- Posaconazole stock solutions for calibration and quality controls
- Drug-free human plasma
- Acetonitrile with 0.5% formic acid (v/v)
- Microcentrifuge tubes, 96-well plates, and a nitrogen evaporator
Procedure:
- Spiking: Pipette 100 µL of plasma sample (calibrator, quality control, or unknown) into a microcentrifuge tube or a 96-well plate.
- Internal Standard Addition: Add 50 µL of itraconazole working solution (prepared in methanol at a concentration appropriate for the intended analytical range) to each sample [34].
- Protein Precipitation: Add 400 µL of ice-cold acetonitrile containing 0.5% formic acid to the sample [34]. This acidified organic solvent ensures efficient protein denaturation and precipitation.
- Mixing and Centrifugation: Vortex-mix the samples vigorously for at least 1 minute. Subsequently, centrifuge at a minimum of 3,500 rpm for 10 minutes to form a compact protein pellet [34] [25].
- Supernatant Transfer: Carefully transfer 400 µL of the clear supernatant to a clean collection plate or tube, avoiding disturbance of the pellet.
- Evaporation and Reconstitution: Evaporate the supernatant to dryness under a gentle stream of nitrogen at 45°C. Reconstitute the dry residue with 100 µL of a mixture of acetonitrile and water (40:60, v/v) [34]. Vortex for 30 seconds to ensure complete dissolution.
- Analysis: The reconstituted sample is now ready for injection into the HPLC or UHPLC system.
Protocol 2: Chromatographic Separation for HPLC-DAD vs. UHPLC-UV
This protocol outlines the distinct conditions for separating posaconazole and itraconazole on both HPLC-DAD and UHPLC-UV platforms, enabling a direct performance comparison [1].
Materials:
- HPLC-DAD System: Agilent 1200 series or equivalent, equipped with a DAD.
- UHPLC-UV System: Agilent 1290 Infinity series or equivalent.
- HPLC Column: Zorbax SB-C18 (4.6 × 250 mm, 5 µm).
- UHPLC Column: Kinetex-C18 (2.1 × 50 mm, 1.3 µm).
- Mobile Phase A: 15 mM potassium dihydrogen orthophosphate in water.
- Mobile Phase B: Acetonitrile (HPLC grade).
Procedure:
- System Setup: Install the appropriate column in the column oven and equilibrate with the starting mobile phase composition.
- Detection Parameters: Set the detection wavelength to 262 nm for both systems, as this is the maximum absorbance wavelength for posaconazole and itraconazole [1].
- Method Execution:
- For HPLC-DAD Analysis: Use a gradient elution starting from 30% B to 80% B over 7 minutes at a flow rate of 1.5 mL/min. The total run time is approximately 11 minutes [1].
- For UHPLC-UV Analysis: Use an isocratic elution with a mobile phase of 45% B and 55% A at a flow rate of 0.4 mL/min. The total run time is significantly shorter, at about 3 minutes [1].
- Injection: Inject 20 µL for HPLC and 5 µL for UHPLC of the reconstituted sample from Protocol 1 [1].
- Data Collection: Monitor the chromatograms and record the retention times and peak areas for both posaconazole and itraconazole (IS).
Table 2: Comparative Chromatographic Conditions for Posaconazole and Itraconazole Separation
| Parameter | HPLC-DAD Method | UHPLC-UV Method |
|---|---|---|
| Analytical Column | Zorbax SB-C18 (4.6 × 250 mm, 5 µm) | Kinetex-C18 (2.1 × 50 mm, 1.3 µm) |
| Mobile Phase | 15 mM KH₂PO₄ : Acetonitrile (Gradient) | 15 mM KH₂PO₄ : Acetonitrile (45:55, Isocratic) |
| Flow Rate | 1.5 mL/min | 0.4 mL/min |
| Injection Volume | 20 µL | 5 µL |
| Run Time | 11 min | 3 min |
| Detection | DAD, 262 nm | UV, 262 nm |
Results and Data Analysis
Method Validation and Performance Data
The method utilizing itraconazole as an internal standard was rigorously validated. The following table summarizes key performance metrics, demonstrating the robustness of the approach.
Table 3: Validation Data for the Quantitation of Posaconazole Using Itraconazole as IS
| Validation Parameter | Result / Value | Experimental Detail |
|---|---|---|
| Linearity Range | 0.1 - 10 µg/mL | Covers the therapeutic range for posaconazole [25]. |
| Correlation Coefficient (r²) | > 0.999 | Observed for both HPLC-DAD and UHPLC-UV methods [1]. |
| Precision (CV%) | < 3% | Intra-day and inter-day precision for both analyte and IS [1]. |
| Accuracy (% Error) | < 3% | Demonstrated across the calibration range [1]. |
| Limit of Quantification (LOQ) | HPLC-DAD: 2.73 µg/mL [1] | UHPLC-UV: 3.16 µg/mL [1] |
| Recovery | > 80% | Consistent recovery for both posaconazole and itraconazole (IS) [33]. |
Internal Standard Selection Justification and Mechanism of Action
The rationale for selecting itraconazole is rooted in its physicochemical properties, which closely mirror those of the analyte, posaconazole.
Logical Relationship Diagram: IS Selection Rationale
- Structural Analogy: Itraconazole is a structural analogue of posaconazole, sharing a triazole core and similar lipophilic side chains [1] [33]. This fundamental similarity ensures that itraconazole will behave almost identically to posaconazole during critical sample preparation steps like protein precipitation, thereby accurately correcting for any analyte loss.
- Chromatographic Coherence: Due to their structural relatedness, itraconazole and posaconazole exhibit similar retention mechanisms on reversed-phase C18 columns. This allows the IS to effectively normalize minor fluctuations in mobile phase composition, flow rate, and column temperature that can cause retention time shifts [1].
- Spectroscopic Compatibility: Both compounds absorb strongly in the UV region, with a characteristic maximum at or near 262 nm [1]. This makes them ideally suited for simultaneous detection using either a DAD or a UV detector, ensuring the IS signal is a reliable proxy for the analyte's detection performance.
Discussion
The integration of itraconazole as an internal standard provides a robust foundation for the precise and accurate quantitation of posaconazole. The experimental data confirms that this approach meets stringent validation criteria for linearity, precision, and accuracy across both HPLC and UHPLC platforms [1]. The primary distinction between the two platforms lies in their operational efficiency. The UHPLC-UV method offers superior performance in terms of analysis speed (3 minutes vs. 11 minutes) and solvent consumption, making it more suitable for high-throughput laboratories [1]. However, the HPLC-DAD method provides the additional advantage of spectral confirmation via the diode array, which can be critical for verifying peak purity and method specificity in complex matrices [36].
The use of a structurally matched internal standard like itraconazole is non-negotiable for high-quality bioanalysis. It systematically corrects for inaccuracies introduced during sample preparation, injection, and analysis, thereby ensuring the reliability of the generated pharmacokinetic data. This validated protocol can be directly adopted for therapeutic drug monitoring and pharmaceutical development studies involving posaconazole and related azole antifungals.
The quantification of posaconazole (PSZ), a broad-spectrum triazole antifungal, is crucial for therapeutic drug monitoring and pharmacokinetic studies, particularly in immunocompromised patients. Method adaptation for the simultaneous quantification of PSZ with other co-administered drugs, such as vincristine (VCR), presents unique analytical challenges that require sophisticated chromatographic solutions. This application note details the development and validation of a robust HPLC-DAD method for the simultaneous quantification of PSZ and VCR in rat plasma, framed within broader thesis research comparing HPLC-DAD with UHPLC-UV platforms [4] [1].
The combination of PSZ with VCR is clinically significant in patients with hematological malignancies, where antifungal prophylaxis is required alongside chemotherapy. However, this co-administration increases the risk of VCR-induced neurotoxicity due to PSZ's inhibition of cytochrome P450 (CYP) isoform 3A4, for which VCR is a substrate [4]. An analytical method capable of simultaneously measuring both drugs from a single blood draw is invaluable for pharmacokinetic drug-drug interaction studies, especially in serial blood collection involving rats where cumulative blood volume withdrawal must be minimized [4].
Experimental Design and Workflow
The simultaneous quantification of analytes with different chemical properties requires careful method adaptation to address extraction efficiency, chromatographic separation, and detection sensitivity challenges in complex biological matrices. The following workflow visualizes the comprehensive experimental approach:
Materials and Reagents
Research Reagent Solutions
Table 1: Essential research reagents and materials for simultaneous quantification
| Item | Specification | Function/Role in Analysis |
|---|---|---|
| Posaconazole | Reference standard, >99% purity | Quantification of target analyte |
| Vincristine sulfate | Reference standard, >99% purity | Quantification of target analyte |
| Itraconazole | Internal standard, pharmaceutical grade | Internal standard for normalization |
| Acetonitrile | HPLC grade | Mobile phase component |
| Methanol | HPLC grade | Solvent for stock solutions |
| Potassium dihydrogen orthophosphate | Analytical grade | Aqueous buffer component |
| Diethyl ether | Analytical grade | Liquid-liquid extraction solvent |
| Sodium hydroxide | Analytical grade | Plasma sample alkalinization |
| C18 Column | 250 × 4.6 mm, 5 µm particle size | Stationary phase for separation |
Instrumentation
The HPLC-DAD system consisted of Agilent 1200 series components, including an autosampler, quaternary pump, vacuum degasser, and diode array detector (G1315 C/D and G1365 C/D) connected to a computer loaded with Agilent ChemStation Software [4]. Separation was achieved using an HC-C18 (4.6 × 250 mm, 5 µm particle size) column attached to an HC-C18 (4.6 × 12.5 mm, 5 µm particle size) guard column [4].
Detailed Experimental Protocols
Sample Preparation Protocol
- Plasma Aliquot: Transfer 200 µL of rat plasma into a glass test tube [4].
- Internal Standard Addition: Add 30 µL of itraconazole (10 mg/L working solution) to each plasma sample [4].
- Alkalinization: Add 30 µL of 0.1 M NaOH solution to adjust pH for optimal extraction [4].
- Liquid-Liquid Extraction: Add 6 mL of diethyl ether to each tube [4].
- Mixing and Phase Separation: Vortex-mix for 2 minutes at high speed, then centrifuge for 10 minutes at approximately 2500 ×g [4].
- Organic Layer Transfer: Transfer the organic layer to new glass tubes [4].
- Evaporation: Evaporate to dryness in vacuo using a rotational vacuum concentrator [4].
- Reconstitution: Reconstitute the dried residue in 100 µL methanol [4].
- Injection: Inject 80 µL volumes into the HPLC system [4].
Chromatographic Conditions Protocol
- Column Equilibration: Pre-condition the C18 column with initial mobile phase composition (30:70 acetonitrile-to-buffer) for at least 30 minutes [4].
- Mobile Phase Preparation:
- Prepare 0.015 M potassium dihydrogen orthophosphate aqueous solution
- Filter both aqueous and organic components through 0.45 µm membrane filters
- Degas by sonication for 10 minutes before use [4]
- Gradient Program:
- Initial: 30% acetonitrile, 70% buffer
- Linear gradient to 80% acetonitrile, 20% buffer over 7 minutes
- Return to initial conditions and re-equilibrate for 5 minutes [4]
- Detection Wavelengths:
- Set DAD to monitor 220 nm for VCR detection
- Set DAD to monitor 262 nm for PSZ detection [4]
- Flow Rate and Temperature: Maintain 1.5 mL/min flow rate and 25°C column temperature throughout analysis [4].
Calibration Standards Preparation
- Stock Solutions: Prepare 100 mg/L stock solutions of PSZ and VCR separately in methanol [4].
- Working Solutions: Prepare fresh working standard solutions (10, 1, and 0.1 mg/L) by successive 1/10 dilutions of stock solutions with methanol [4].
- Calibration Curve: Prepare samples in 0.2 mL rat plasma spanning 50-5000 ng/mL for both PSZ and VCR [4].
- Quality Controls: Prepare QC samples at low, medium, and high concentrations (50, 500, and 2500 ng/mL) for validation [4].
Results and Data Analysis
Method Validation Parameters
Table 2: Comprehensive method validation results for simultaneous quantification
| Validation Parameter | Posaconazole | Vincristine | Acceptance Criteria |
|---|---|---|---|
| Linear Range (ng/mL) | 50-5000 | 50-5000 | - |
| Correlation Coefficient (r²) | >0.999 | >0.999 | r² ≥ 0.99 |
| Limit of Quantification (ng/mL) | 50 | 50 | S/N ≥ 10 |
| Intra-day Precision (%CV) | 2.77-5.93 | ≤18 | ≤15% |
| Inter-day Precision (%CV) | ≤15 | ≤18 | ≤15% |
| Accuracy (% Bias) | -2.48 to 3.70 | -8.10 to 3.77 | ±15% |
| Recovery (%) | >80 | >80 | Consistent and reproducible |
| Analytical Run Time (min) | 11 | 11 | - |
Chromatographic Performance
The optimized method successfully separated PSZ, VCR, and itraconazole (IS) within 11 minutes [4]. PSZ and VCR were measured at 262 nm and 220 nm, respectively, with baseline resolution and no interference from plasma components [4]. The retention times were reproducible with %RSD < 2%, demonstrating excellent method robustness [4].
The recovery of PSZ, VCR, and ITZ was determined at 2500 ng/mL concentration level in rat plasma using four replicates for each concentration [4]. The extraction efficiency was determined by comparing the peak areas of each analyte to the peak areas of the same amounts directly injected to the instrument without extraction, demonstrating excellent recovery rates above 80% for both analytes [4].
HPLC-DAD versus UHPLC-UV Comparative Analysis
Table 3: Technical comparison between HPLC-DAD and UHPLC-UV platforms
| Parameter | HPLC-DAD Method | UHPLC-UV Method |
|---|---|---|
| Analysis Time | 11 minutes | 3 minutes |
| Column Dimensions | 250 × 4.6 mm, 5 µm | 50 × 2.1 mm, 1.3 µm |
| Flow Rate | 1.5 mL/min | 0.4 mL/min |
| Injection Volume | 80 µL | 5 µL |
| Mobile Phase | Gradient: 30:70 to 80:20 acetonitrile:buffer | Isocratic: 45:55 acetonitrile:buffer |
| Detection | Diode Array (multiple wavelengths) | UV (262 nm) |
| Theoretical Plates | >2000 | >5000 |
| Solvent Consumption per Run | ~16.5 mL | ~1.2 mL |
| Limit of Quantification | 50 ng/mL (biological samples) | 3.16 µg/mL (pharmaceuticals) |
The comparison reveals that UHPLC-UV offers advantages in analysis speed (3 minutes versus 11 minutes) and solvent consumption reduction (~1.2 mL versus ~16.5 mL per run) [1]. However, the HPLC-DAD method provides superior sensitivity for biological samples (LOQ of 50 ng/mL versus 3.16 µg/mL) and the flexibility of multiple wavelength detection, which is essential for simultaneous quantification of drugs with different absorbance maxima [4] [1].
Troubleshooting and Optimization Guidelines
Common Issues and Solutions
Poor Peak Shape
- Cause: Secondary interactions with stationary phase or column degradation
- Solution: Use fresh mobile phase with appropriate pH control; condition column properly
Retention Time Drift
- Cause: Mobile phase composition or temperature fluctuations
- Solution: Ensure mobile phase equilibration; maintain constant column temperature
Reduced Recovery
- Cause: Inefficient extraction or protein binding
- Solution: Optimize alkalinization; ensure sufficient extraction solvent volume and mixing time
Matrix Interference
- Cause: Endogenous plasma components
- Solution: Optimize sample clean-up; confirm specificity using multiple detection wavelengths
Method Adaptation for Different Matrices
When adapting this method for different biological matrices or additional analytes:
- Extraction Optimization: Screen different extraction solvents (TBME, n-hexane, ethyl acetate) and pH conditions [3]
- Gradient Adjustment: Modify gradient profile to maintain resolution for new analyte combinations
- Detection Wavelength Selection: Identify optimal wavelengths for new analytes using DAD scanning
- Validation Protocol: Re-establish validation parameters according to ICH M10 guidelines [37]
Application in Pharmacokinetic Studies
The validated method was successfully applied to analyze plasma samples from Sprague Dawley rats orally dosed with PSZ (40 mg/kg) followed by intravenous dosing of VCR (0.1 mg/kg) through the tail vein after 30 minutes of oral dosing [4]. Serial blood samples were collected at 0.50, 0.75, 0.92, 1.33, 2.0, 3.5, 6.0, 8.0, 24.0, 48.0, and 72.0 hours after oral PSZ dose using retroorbital sampling [4]. Plasma was separated by centrifugation of the blood at approximately 2500 ×g for 3 minutes, and the samples were kept at -20°C until analysis time [4].
Rat plasma concentrations of PSZ and VCR were simultaneously measured up to 72 hours, and their calculated pharmacokinetics parameters were comparable to literature values [4]. This demonstrates the method's applicability for pharmacokinetic drug-drug interaction studies, providing a valuable tool for investigating the complex interaction between PSZ and VCR, particularly the exacerbation of VCR neurotoxicity when co-administered with azole antifungals [4].
This application note provides a detailed protocol for the simultaneous quantification of posaconazole and vincristine in rat plasma using HPLC-DAD. The method demonstrates excellent linearity, precision, accuracy, and sensitivity across the clinically relevant concentration range of 50-5000 ng/mL for both analytes. The successful application in a pharmacokinetic study highlights the method's robustness for drug-drug interaction investigations.
The comparison with UHPLC-UV reveals a trade-off between analysis speed and detection flexibility, with HPLC-DAD offering advantages for simultaneous multi-analyte quantification in complex matrices. This methodology provides researchers with a validated framework for therapeutic drug monitoring and pharmacokinetic studies of this critical drug combination, with potential for adaptation to other analyte pairs requiring simultaneous quantification.
Optimizing Chromatographic Performance and Overcoming Common Challenges
Column Chemistry and Mobile Phase Optimization for Peak Symmetry and Resolution
Within the framework of research quantifying the antifungal drug posaconazole, achieving optimal peak symmetry and resolution is not merely a methodological preference but a fundamental requirement for generating reliable, reproducible, and accurate data. The comparative analysis of High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) and Ultra-High-Performance Liquid Chromatography with Ultraviolet Detection (UHPLC-UV) presents distinct challenges and opportunities for method optimization [1]. This application note provides a detailed, practical guide for researchers and drug development professionals, contextualized within posaconazole quantitation studies. We summarize critical experimental data, provide step-by-step protocols for key experiments, and visualize the optimization workflow to facilitate robust analytical method development.
Comparative Methodologies: HPLC-DAD vs. UHPLC-UV for Posaconazole
The selection of chromatographic technique directly influences the efficiency, solvent consumption, and speed of analysis. A direct comparison of methods developed for posaconazole suspension illustrates the core trade-offs.
Table 1: Comparative Chromatographic Conditions for Posaconazole Quantitation
| Parameter | HPLC-DAD Method | UHPLC-UV Method |
|---|---|---|
| Stationary Phase | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) [1] | Kinetex-C18 (2.1 × 50 mm, 1.3 μm) [1] |
| Mobile Phase | Gradient: Acetonitrile : 15 mM KH₂PO₄ (30:70 to 80:20) [1] | Isocratic: Acetonitrile : 15 mM KH₂PO₄ (45:55) [1] |
| Flow Rate | 1.5 mL/min [1] | 0.4 mL/min [1] |
| Run Time | 11 minutes [1] | 3 minutes [1] |
| Injection Volume | 20-50 μL [1] | 5 μL [1] |
| Column Temperature | 25 °C [1] | 40 °C [1] |
Table 2: Analytical Performance Data for Posaconazole Methods
| Performance Metric | HPLC-DAD Method | UHPLC-UV Method |
|---|---|---|
| Linearity Range | 5–50 μg/mL [1] | 5–50 μg/mL [1] |
| Correlation Coefficient (r²) | >0.999 [1] | >0.999 [1] |
| Limit of Detection (LOD) | 0.82 μg/mL [1] | 1.04 μg/mL [1] |
| Limit of Quantitation (LOQ) | 2.73 μg/mL [1] | 3.16 μg/mL [1] |
| Precision (CV%) | <3% [1] | <3% [1] |
The UHPLC method demonstrates clear advantages in analysis speed and solvent economy, achieved through a combination of a smaller-particle-size column, elevated temperature, and a reduced flow rate [1]. The UHPLC-UV assay exhibited some economic and chromatographic separation superiority, making it particularly suitable for high-throughput quality control environments [1].
Core Principles of Optimization for Peak Symmetry and Resolution
Peak symmetry and resolution are governed by the interplay of column chemistry, mobile phase composition, and instrument parameters. The fundamental goal is to maximize the interaction of analytes with the stationary phase in a controlled manner to separate components.
Column Chemistry and Selectivity
The stationary phase is the primary determinant of selectivity.
- Stationary Phase Chemistry: While a C18 column is a common starting point, exploring alternative phases can dramatically improve resolution for challenging separations. Embedded polar group phases (e.g., amide, carbamate) can offer superior selectivity and retention for polar compounds compared to traditional alkyl, cyano, or phenyl phases [38]. These phases are often orthogonal to C18, meaning they produce a different elution order, which can resolve co-eluting peaks [38].
- Particle Size and Morphology: Smaller particles (e.g., sub-2 μm in UHPLC) increase column efficiency (theoretical plates, N), leading to sharper peaks and better resolution [1] [39]. Solid-core particles can further enhance efficiency and allow for higher flow rates without excessive backpressure [39].
- Column Dimensions: Longer columns generally improve resolution but increase backpressure and analysis time [39]. Shorter columns packed with smaller particles are ideal for fast, efficient separations, as demonstrated in the UHPLC method for posaconazole [1].
Mobile Phase Optimization
The mobile phase is a powerful tool for manipulating retention and peak shape.
- pH and Buffer Strength: Mobile phase pH can significantly impact the ionization state of ionizable analytes, thereby affecting retention and peak shape. Using buffers with appropriate ionic strength (e.g., 15 mM potassium dihydrogen orthophosphate) helps control ionization and improves peak symmetry [1] [40]. For instance, the analysis of quercetin was optimized using a mobile phase acidified with 1.5% acetic acid [40].
- Organic Modifier and Gradient Elution: The type and ratio of organic solvent (e.g., acetonitrile, methanol) control elution strength. Isocratic elution is simple but may not resolve complex mixtures. Gradient elution, where the organic proportion increases over time, is advantageous for samples with a wide range of hydrophobicity, as it compresses later-eluting peaks, improving sensitivity and reducing run time [1] [38].
- Temperature Optimization: Higher column temperatures reduce mobile phase viscosity, allowing faster flow rates and lower backpressure. They can also improve mass transfer, leading to better efficiency and peak shape [39]. However, very high temperatures may degrade the sample or column. The UHPLC method for posaconazole used a column temperature of 40°C to enhance performance [1].
Experimental Protocols
Protocol 1: Systematic Method Scouting for Column and Mobile Phase Selection
This protocol is designed for the initial development of a chromatographic method for a small molecule like posaconazole.
1. Preparation of Standard and Mobile Phases:
- Prepare a stock solution of the analyte (e.g., 100 μg/mL of posaconazole) in a suitable solvent (e.g., methanol) [1].
- Prepare a set of candidate mobile phases. Example A: 15 mM potassium dihydrogen phosphate buffer. Example B: Acetonitrile. Example C: Methanol. Example D: Acidified water (e.g., 1.5% acetic acid) [1] [40].
2. Column Screening and Initial Conditions:
- Select 2-3 columns with different selectivities (e.g., C18, RP-amide, fluorinated) [38].
- Set the detector wavelength based on the analyte's UV spectrum (e.g., 262 nm for posaconazole) [1].
- Begin with an isocratic method of 50% organic modifier (B) and 50% aqueous phase (A) at a flow rate of 0.3-0.4 mL/min for UHPLC or 1.0-1.5 mL/min for HPLC [1] [41].
- Set the column temperature to 35-40°C [1].
- Inject 1-5 μL of the standard solution [1].
3. Gradient Scouting and Optimization:
- If peaks are poorly resolved, switch to a broad gradient (e.g., 5% to 95% B over 10 minutes).
- Observe the elution pattern. The goal is to achieve a resolution (Rs) of >1.5 between all critical peak pairs.
- Based on the scouting run, adjust the gradient profile to fine-tune separation. If all peaks elute early, use a weaker gradient. If they elute late, use a stronger gradient [38].
4. Isocratic Fine-Tuning and Finalization:
- Based on the gradient results, transition back to an isocratic method using a %B value that places the peaks of interest in a retention factor (k) range of 1-10 [38].
- Make small adjustments (± 2-5%) to the organic modifier to optimize resolution.
- Adjust the mobile phase pH in 0.1-0.2 unit increments to improve the separation of ionizable compounds and peak symmetry [39] [40].
Protocol 2: Method Optimization for Peak Symmetry and Resolution
Once a preliminary method is established, this protocol focuses on refining peak shape and resolution.
1. Assessment of Initial Chromatogram:
- Inject the standard and calculate the asymmetry factor (As) for the main peak. A value between 0.9 and 1.5 is generally acceptable.
- Calculate the resolution (Rs) between the most poorly separated peak pair.
2. Optimization of Flow Rate and Temperature:
- If peaks are broad or asymmetric, systematically adjust the flow rate. A lower flow rate can improve mass transfer and peak shape but increases run time [39].
- Increase the column temperature in 5°C increments (up to the column's limit, typically 60°C) to enhance efficiency and improve peak shape. Monitor for any signs of degradation [39].
3. Addressing Peak Tailing:
- Significant peak tailing (As > 1.5) often indicates secondary interactions with active sites on the stationary phase.
- Increase the buffer concentration (e.g., from 15 mM to 25 mM) to better mask these silanol groups [1] [39].
- Consider using a mobile phase additive like formic acid or ammonium formate for improved performance in LC-MS applications [41].
4. Final Method Validation:
- Once optimal conditions are found, validate the method according to ICH guidelines for linearity, precision, accuracy, LOD, and LOQ [1] [40].
- The method should be tested on the actual sample matrix (e.g., posaconazole suspension) to ensure no interferences [1].
Diagram 1: A systematic workflow for developing and optimizing an HPLC/UHPLC method to achieve baseline resolution and symmetric peaks.
The Scientist's Toolkit: Research Reagent Solutions
The following table details essential materials and their functions for developing and executing chromatographic methods for drug quantitation.
Table 3: Essential Research Reagents and Materials for HPLC/UHPLC Method Development
| Item | Function & Application | Example from Literature |
|---|---|---|
| C18 Chromatography Columns | Reversed-phase separation based on hydrophobicity; the workhorse for most small-molecule drugs. | Zorbax SB-C18 (HPLC), Kinetex-C18 (UHPLC) for posaconazole [1]. |
| Embedded Polar Group Phases | Provides orthogonal selectivity for polar compounds; reduces peak tailing for basic analytes. | RP-Amide phase for improved resolution of polar catechols [38]. |
| Potassium Dihydrogen Phosphate (KH₂PO₄) | Common buffer salt for controlling mobile phase pH and ionic strength to improve peak shape. | Used in 15 mM concentration for posaconazole mobile phase [1]. |
| HPLC-Grade Acetonitrile and Methanol | Primary organic modifiers in the mobile phase to control elution strength and selectivity. | Acetonitrile used in both HPLC and UHPLC posaconazole methods [1]. |
| Acetic Acid / Formic Acid | Mobile phase additives to acidify the eluent, suppressing analyte ionization for better retention and peak symmetry. | 1.5% acetic acid for quercetin analysis [40]; ammonium formate for valsartan/nifedipine [41]. |
| Analytical Standard (Pure Analytic) | Required for preparing calibration standards for method validation and quantitative analysis. | Pure posaconazole powder for stock solution [1]. |
| Internal Standard | Compound added to samples to correct for variability in injection volume and sample processing. | Itraconazole used as IS for posaconazole quantitation [1]. |
Optimizing column chemistry and mobile phase composition is a systematic process that directly dictates the success of quantitative analysis, as exemplified in the comparative study of posaconazole. By understanding the principles of selectivity, leveraging modern stationary phases, and meticulously adjusting mobile phase parameters, researchers can achieve the peak symmetry and resolution necessary for robust and reliable data. The protocols and guidelines provided here offer a concrete pathway for scientists to enhance their analytical methods, ensuring accuracy and efficiency in pharmaceutical development and quality control.
Advanced Chemometric and Machine Learning Approaches for Parameter Optimization
The quantitative analysis of pharmaceutical compounds like posaconazole, a broad-spectrum triazole antifungal agent, relies heavily on robust and efficient chromatographic methods [1] [3]. High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) and Ultra-High-Performance Liquid Chromatography with UV detection (UHPLC-UV) are cornerstone techniques for this purpose, playing a critical role in quality control, formulation assessment, and pharmacokinetic studies [1] [2] [21]. The performance of these methods is profoundly influenced by multiple interacting parameters, making their optimization a complex but essential process.
Traditional one-variable-at-a-time (OVAT) optimization approaches are not only time-consuming and resource-intensive but also often fail to identify optimal conditions due to their inability to account for factor interactions [3]. This review explores the transformative role of advanced chemometric and machine learning (ML) approaches in overcoming these limitations for the optimization of HPLC-DAD and UHPLC-UV methods, with a specific focus on posaconazole quantification. These data-driven strategies enable a more systematic, efficient, and in-depth exploration of the experimental parameter space, leading to enhanced analytical performance, reduced method development time, and more robust and reliable quantification methods [42] [3].
Chemometric Designs for Systematic Parameter Optimization
Chemometric experimental designs provide a structured framework for simultaneously investigating the effects of multiple factors and their interactions on chromatographic responses with a minimal number of experimental runs.
Factorial Designs for Screening and Optimization
Factorial designs are powerful tools for identifying which factors among many have significant effects on the analytical method's performance. In the context of developing an HPLC-UV method for posaconazole quantification in low-volume plasma samples, a fractional 2-level factorial design was successfully employed to evaluate five critical factors: total organic phase percentage, methanol percentage in the organic phase, mobile phase pH, column temperature, and flow rate [3]. This design allowed the researchers to efficiently screen these parameters and understand their influence on retention time and peak resolution. Similarly, the development of a rapid UHPLC-UV method for posaconazole in bulk and suspension forms demonstrated the advantage of systematic optimization, achieving an impressive run time of just 3 minutes [1].
Response Surface Methodology for Final Optimization
After identifying significant factors through screening designs, Response Surface Methodology (RSM) is used to locate the precise optimum conditions. Central Composite Design (CCD) and Box-Behnken Design are the most common RSM approaches. These designs model the relationship between factors and responses, enabling the prediction of optimal chromatographic conditions that might not be intuitively obvious. The application of these designs leads to methods with superior performance characteristics, including enhanced sensitivity, resolution, and efficiency [43].
Table 1: Key Parameters Optimized for Posaconazole Quantification
| Analytical Technique | Critical Parameters Optimized | Optimization Approach | Key Outcome | Reference |
|---|---|---|---|---|
| HPLC-UV (Plasma Analysis) | Organic phase %, MeOH %, pH, temperature, flow rate | Fractional Factorial Design + Machine Learning | Runtime: 8.2 min; LOQ: 50 ng/mL | [3] |
| UHPLC-UV (Bulk/Suspension) | Column type, mobile phase gradient, flow rate | Methodical comparison and optimization | Runtime: 3 min; Linear range: 5–50 μg/mL | [1] |
| HPLC (Bulk/Tablets) | Mobile phase composition, column type, wavelength | Validation per ICH guidelines | Linear range: 2–20 μg/mL; Recovery: ~99% | [2] [21] |
Machine Learning Models for Predictive Optimization
Machine learning algorithms represent a significant advancement beyond traditional chemometrics, offering powerful predictive modeling and pattern recognition capabilities for chromatographic optimization.
Integration of ANN with Experimental Design
A novel approach combined a fractional factorial design with an Artificial Neural Network (ANN) to optimize both the chromatographic separation and extraction of posaconazole from plasma [3]. The factorial design provided the initial dataset, which was then used to train the ANN model. The ANN, known for its ability to model complex non-linear relationships, learned the intricate connections between the input parameters (e.g., organic phase composition, pH) and the output responses (retention time, recovery). Once trained, the ANN could accurately predict outcomes for new combinations of parameters, significantly reducing the need for extensive experimental trials.
Ensemble Learning for Complex Data
Ensemble learning methods, such as Random Forest and Extremely Randomized Trees (Extratrees), are particularly well-suited for handling complex, high-dimensional data, such as UV-VIS spectral libraries for compound identification in HPLC-DAD [42]. These algorithms operate by constructing a multitude of decision trees during training and outputting a consensus prediction, which enhances generalization and robustness compared to a single model. The "bagging" (bootstrap aggregating) strategy used in Random Forest mitigates the risk of overfitting and improves prediction accuracy, making it a valuable tool for modeling chromatographic behavior and recognizing spectral patterns despite variations in experimental conditions [42].
The following diagram illustrates the integrated workflow of chemometric designs and machine learning models for analytical method optimization.
Experimental Protocols
Protocol: Optimizing Chromatographic Conditions Using Factorial Design and ANN
This protocol details the steps for optimizing an HPLC method for posaconazole quantification in plasma, integrating experimental design with machine learning [3].
- Define Factors and Responses: Identify critical parameters to optimize. For chromatography, these may include organic phase percentage (A), methanol percentage in organic phase (B), mobile phase pH (C), column temperature (D), and flow rate (E). Key responses are retention time (RT) of posaconazole and internal standard, and peak resolution [3].
- Design Experimental Matrix: Create a fractional 2-level factorial design (e.g., 16 runs with 4 center points) using statistical software. The design should randomize the run order to minimize bias.
- Execute Experiments: Perform the HPLC runs according to the designed matrix. Use a suitable C8 or C18 column and a UV detector set at the λmax of posaconazole (e.g., 262 nm). Record the RT and peak data for all responses [1] [3].
- Build and Train the ANN Model: Input the experimental data into an ANN platform. The network architecture typically consists of an input layer (factors), one or more hidden layers, and an output layer (responses). Train the network using a suitable algorithm (e.g., backpropagation) to minimize the error between predicted and actual values.
- Predict and Validate Optimum: Use the trained ANN model to predict the combination of factors that will yield the desired responses (e.g., shortest RT with good resolution). Experimentally validate the predicted optimum conditions to confirm the model's accuracy.
Protocol: Developing a Validated UHPLC-UV Method for Posaconazole Formulations
This protocol describes the development and validation of a fast UHPLC-UV method for quality control of posaconazole in suspension and bulk forms [1] [2].
- Column and Mobile Phase Selection: Select a UHPLC column with sub-2µm particles (e.g., Kinetex-C18, 2.1 × 50 mm, 1.3 µm). Prepare a mobile phase of acetonitrile and 15 mM potassium dihydrogen orthophosphate. An isocratic elution (e.g., 45:55) or a fast gradient can be used [1].
- System Setup and Equilibration: Set the flow rate to 0.4 mL/min, column temperature to 40°C, and detection wavelength to 262 nm. Inject a small volume (e.g., 5 µL). Allow the system to equilibrate before analysis.
- Standard and Sample Preparation:
- Stock Solution: Accurately weigh posaconazole and dissolve in methanol to prepare a 100 µg/mL stock solution [1].
- Calibration Standards: Prepare serial dilutions in methanol covering the range of 5–50 µg/mL.
- Suspension Sample: Dilute the oral suspension with methanol, centrifuge, and dilute the supernatant further with methanol to fall within the calibration range [1].
- Method Validation: Validate the method according to ICH guidelines [2] [21].
- Linearity: Inject each calibration standard in triplicate. Plot peak area vs. concentration and calculate the correlation coefficient (r² > 0.999).
- Precision: Assess intra-day precision by analyzing three concentrations (e.g., 5, 20, 50 µg/mL) in replicates within the same day. Assess inter-day precision over three consecutive days. Calculate the % relative standard deviation (%RSD), which should be < 2% [1] [2].
- Accuracy: Perform a recovery study by spiking a known amount of posaconazole standard into the placebo or pre-analyzed sample. The percentage recovery should be close to 100% [2] [21].
- Specificity: Confirm that the posaconazole peak is pure and free from interference from excipients or degradation products by analyzing blank and sample solutions [21].
Table 2: Summary of Method Validation Data for Posaconazole HPLC/UHPLC Methods
| Validation Parameter | HPLC-DAD Method [1] | UHPLC-UV Method [1] | HPLC Method [2] [21] |
|---|---|---|---|
| Linearity Range | 5–50 μg/mL | 5–50 μg/mL | 2–20 μg/mL |
| Correlation (r²) | > 0.999 | > 0.999 | Not Specified |
| Precision (%RSD) | < 3% | < 3% | < 1% |
| LOD | 0.82 μg/mL | 1.04 μg/mL | Not Specified |
| LOQ | 2.73 μg/mL | 3.16 μg/mL | Not Specified |
| Run Time | 11 min | 3 min | Not Specified |
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for Posaconazole HPLC/DAD Analysis
| Item | Specification / Example | Function in the Analysis |
|---|---|---|
| Posaconazole Reference Standard | High-purity (e.g., ≥ 98%) from certified suppliers [2] [3] | Primary standard for calibration and quantification. |
| HPLC-Grade Acetonitrile & Methanol | Low UV cutoff, high purity [1] [2] | Mobile phase components for compound separation. |
| Buffer Salts | Potassium dihydrogen orthophosphate [1] | Provides aqueous component of mobile phase; controls pH. |
| Water | HPLC-grade deionized water (18 MΩ·cm) [2] | Mobile phase component and solvent for aqueous solutions. |
| Analytical Column | C18 (e.g., Zorbax SB-C18, 5µm) for HPLC; sub-2µm C18 for UHPLC [1] | Stationary phase for chromatographic separation of analytes. |
| Syringe Filters | 0.22 μm or 0.45 μm PVDF or Nylon [2] [44] | Removal of particulate matter from samples prior to injection. |
The integration of chemometrics and machine learning with chromatographic science marks a paradigm shift in analytical method development. As demonstrated in the optimization of posaconazole quantification methods, these approaches facilitate a more efficient, in-depth, and predictive understanding of the complex relationships between chromatographic parameters and analytical outcomes. The move from traditional OVAT to multivariate, data-driven strategies enables the development of more robust, sensitive, and faster HPLC-DAD and UHPLC-UV methods. This is crucial not only for the quality control of posaconazole but also for advancing pharmaceutical analysis broadly, ultimately contributing to more reliable drug efficacy and safety profiling. Future advancements will likely see a deeper integration of AI, making the optimization process even more intelligent and autonomous.
Strategies to Enhance Sensitivity and Reduce Limits of Quantification (LOQ)
Within the context of analytical method development for the quantitation of posaconazole, the choice between High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) and Ultra-High-Performance Liquid Chromatography with Ultraviolet Detection (UHPLC-UV) is pivotal. A primary objective in this domain is the enhancement of method sensitivity and the reduction of the Limits of Detection (LOD) and Quantification (LOQ). These parameters are critical for supporting pharmacokinetic studies, therapeutic drug monitoring, and quality control of pharmaceutical products, especially given the variable bioavailability of posaconazole formulations [3] [21]. This document outlines validated strategies and protocols to achieve these goals, providing a direct comparison of the performance characteristics of HPLC-DAD and UHPLC-UV methods.
Comparative Method Performance: HPLC-DAD vs. UHPLC-UV
The fundamental differences in column technology and system pressure between HPLC and UHPLC directly influence key sensitivity parameters. The table below summarizes the performance data from a direct comparative study of the two techniques for posaconazole analysis [1] [45].
Table 1: Comparative analytical performance of HPLC-DAD and UHPLC-UV methods for posaconazole.
| Parameter | HPLC-DAD Method | UHPLC-UV Method |
|---|---|---|
| Linearity Range | 5–50 μg/mL | 5–50 μg/mL |
| Correlation Coefficient (r²) | >0.999 | >0.999 |
| Limit of Detection (LOD) | 0.82 μg/mL | 1.04 μg/mL |
| Limit of Quantification (LOQ) | 2.73 μg/mL | 3.16 μg/mL |
| Run Time | 11 minutes | 3 minutes |
| Precision (CV%) & Accuracy (% Error) | <3% | <3% |
| Mobile Phase Consumption | Higher (1.5 mL/min flow rate) | Lower (0.4 mL/min flow rate) |
While the cited study shows a marginally better LOD/LOQ for the HPLC-DAD method, UHPLC demonstrates significant advantages in analysis speed and solvent economy, which are crucial for high-throughput laboratories [1]. It is important to note that other studies utilizing advanced optimization techniques have achieved substantially lower LOQs, for example, down to 50 ng/mL (0.05 μg/mL) in biological matrices, showcasing the potential for sensitivity enhancement in both platforms [3] [4].
Experimental Protocols for Enhanced Sensitivity
Protocol 1: UHPLC-UV Method for Pharmaceutical Dosage Forms
This protocol is optimized for rapid analysis with low solvent consumption, suitable for quality control of bulk powder and suspension dosage forms [1].
3.1.1 Equipment and Reagents:
- Chromatograph: UHPLC system capable of handling high backpressures.
- Column: Kinetex C18 (2.1 × 50 mm, 1.3 μm).
- Mobile Phase: Acetonitrile and 15 mM Potassium dihydrogen orthophosphate (55:45, v/v).
- Standard Solution: Posaconazole stock solution in methanol (100 μg/mL).
- Internal Standard: Itraconazole (10 μg/mL in methanol).
3.1.2 Procedure:
- Mobile Phase Preparation: Prepare 15 mM potassium dihydrogen orthophosphate in HPLC-grade water. Mix with HPLC-grade acetonitrile in a 45:55 (buffer:acetonitrile) ratio. Filter through a 0.22 μm membrane and degas by sonication.
- Standard Curve Preparation: Spike posaconazole into methanol to create concentrations from 5-50 μg/mL. Add a fixed concentration of itraconazole (10 μg/mL) as the internal standard.
- Sample Preparation (Suspension): Dilute 0.1 mL of posaconazole oral suspension (40 mg/mL) to 10 mL with methanol. Further dilute this solution with methanol, adding the internal standard, to a final volume of 1 mL.
- Chromatographic Conditions:
- Flow Rate: 0.4 mL/min
- Injection Volume: 5 μL
- Column Temperature: 40 °C
- Detection Wavelength: 262 nm
- Elution Mode: Isocratic
- Analysis: Inject standards and samples. Plot the peak area ratio (posaconazole/internal standard) against concentration to generate the calibration curve.
Protocol 2: HPLC-UV Method for Low-Volume Plasma Samples
This protocol employs a robust sample preparation and chemometric optimization to achieve a low LOQ (50 ng/mL) in low-volume plasma, ideal for preclinical pharmacokinetic studies [3].
3.2.1 Equipment and Reagents:
- Chromatograph: HPLC system with UV detector.
- Column: PerfectSil Target C8 (250 × 4.6 mm, 5 μm).
- Mobile Phase: Combination of Acetonitrile and Methanol with a pH 7 aqueous buffer.
- Extraction Solvent: Tertiary butyl methyl ether (TBME).
- Internal Standard: Diazepam.
3.2.2 Procedure:
- Optimized Chromatography Conditions:
- Total Organic Phase: 58%
- Methanol in Organic Phase: 6%
- Mobile Phase pH: 7
- Column Temperature: 39 °C
- Flow Rate: 1.2 mL/min
- Detection: 262 nm
- Plasma Sample Extraction:
- To a 100 μL aliquot of spiked rat plasma, add the internal standard (Diazepam).
- Add 500 μL of TBME extraction solvent and 10% w/v sodium chloride. Adjust plasma pH to 11.
- Vortex-mix for 10 minutes and centrifuge at high speed for 1 minute.
- Transfer the organic layer and evaporate to dryness under a gentle stream of nitrogen.
- Reconstitute the dry residue with 100 μL of the mobile phase and inject into the HPLC system.
- Analysis: Construct the calibration curve in the range of 50–2000 ng/mL using the peak area ratio (posaconazole/internal standard).
- Optimized Chromatography Conditions:
Workflow for Sensitivity-Optimized Method Development
The following diagram illustrates a systematic workflow for developing a sensitive LC-UV method, integrating strategies from the cited research.
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table lists key reagents and materials critical for implementing the described protocols and achieving high sensitivity in posaconazole quantification.
Table 2: Key research reagents and materials for posaconazole HPLC/UHPLC analysis.
| Item | Function / Role | Example from Protocols |
|---|---|---|
| C18 or C8 Column | Stationary phase for reverse-phase separation; smaller particles (e.g., 1.3 μm) enhance efficiency and speed in UHPLC. | Kinetex C18 (1.3 μm) [1], PerfectSil Target C8 (5 μm) [3] |
| Acetonitrile & Methanol | Organic modifiers in the mobile phase; composition and ratio critically impact retention time and peak shape. | HPLC grade [1] [3] [21] |
| Potassium Dihydrogen Phosphate / Phosphate Buffer | Aqueous component of mobile phase; buffer concentration and pH (e.g., pH 7) are key for controlling ionization and reproducibility. | 15 mM, pH adjusted [1] [4] |
| Internal Standard (IS) | Compound added to correct for variability in sample preparation and injection; should be structurally similar. | Itraconazole [1] [4], Diazepam [3] |
| Liquid-Liquid Extraction (LLE) Solvent | Used to extract analyte from complex matrices (e.g., plasma), removing proteins and interfering substances. | Tertiary Butyl Methyl Ether (TBME) [3], Diethyl Ether [4] |
| Protein Precipitation Solvent | Alternative to LLE; acetonitrile or methanol is added to plasma to precipitate proteins, followed by centrifugation. | Acetonitrile [46] |
Enhancing sensitivity and reducing LOQ in posaconazole quantitation is a multi-faceted endeavor. The direct comparison demonstrates that while HPLC-DAD can offer marginal gains in LOD/LOQ for specific methods, UHPLC-UV provides superior speed and operational economy [1]. The most significant sensitivity improvements are achieved through a holistic strategy: employing advanced column chemistries with smaller particle sizes, optimizing mobile phase composition and pH using statistical experimental design, and implementing clean, efficient sample preparation techniques like liquid-liquid extraction [3]. The provided protocols and workflow offer a robust foundation for researchers to develop fit-for-purpose methods that meet the stringent sensitivity requirements for modern pharmaceutical analysis and therapeutic drug monitoring of posaconazole.
Mitigating Matrix Effects and Interferences in Biological Samples
Matrix effects represent a significant challenge in the bioanalytical quantification of pharmaceuticals, particularly when using liquid chromatography coupled with ultraviolet or diode array detection (HPLC-DAD/UV). These effects are defined as the combined influence of all sample components other than the analyte on the measurement of the quantity [47]. In the specific context of posaconazole therapeutic drug monitoring, matrix effects can substantially impact accuracy, precision, and sensitivity, potentially compromising clinical decision-making [1] [21].
The fundamental problem arises because biological matrices such as plasma, blood, and urine contain numerous endogenous compounds—including proteins, phospholipids, and salts—that can co-elute with the target analyte, leading to signal suppression or enhancement [47] [48]. When developing methods for posaconazole quantification using HPLC-DAD and UHPLC-UV, understanding and mitigating these matrix interferences becomes paramount for obtaining reliable analytical results that can support pharmacokinetic studies and therapeutic drug monitoring protocols [1] [21].
Evaluating Matrix Effects: Strategic Approaches
Before implementing mitigation strategies, researchers must first assess the presence and extent of matrix effects. Several well-established experimental approaches facilitate this evaluation, each providing distinct but complementary information.
Table 1: Methods for Evaluating Matrix Effects
| Method Name | Description | Type of Assessment | Key Limitations |
|---|---|---|---|
| Post-Column Infusion [47] | Continuous infusion of analyte into HPLC effluent while injecting blank matrix extract; identifies regions of ion suppression/enhancement. | Qualitative | Does not provide quantitative data; labor-intensive for multiple analytes. |
| Post-Extraction Spike [47] | Comparison of analyte response in neat solution versus analyte spiked into blank matrix after extraction. | Quantitative | Requires access to blank matrix. |
| Slope Ratio Analysis [47] | Comparison of calibration curve slopes in neat solution versus matrix across a concentration range. | Semi-quantitative | Requires multiple concentration levels. |
Post-Column Infusion Methodology
The post-column infusion method provides a qualitative assessment of matrix effects, identifying retention time zones most susceptible to ion enhancement or suppression [47].
Experimental Protocol:
- Prepare a blank matrix extract by processing drug-free biological matrix (e.g., plasma) through the entire sample preparation procedure.
- Configure the HPLC system with a T-connector between the column outlet and detector.
- Establish chromatographic conditions for posaconazole separation (e.g., C18 column, mobile phase: acetonitrile:15 mM potassium dihydrogen phosphate, detection: 262 nm) [1].
- Infuse a posaconazole standard solution (e.g., 1-5 μg/mL) at a constant flow rate (e.g., 10-20 μL/min) via the T-connector.
- Inject the blank matrix extract while monitoring the analyte signal.
- Record regions of signal fluctuation, noting retention times where suppression or enhancement exceeds ±15%.
This method successfully identified variable matrix effects across different biological matrices in a study of 33 pharmaceuticals, highlighting the importance of matrix-specific method optimization [47].
Post-Extraction Spiking Method
For quantitative assessment of matrix effects, the post-extraction spiking method provides a straightforward approach to calculate absolute matrix effect.
Experimental Protocol:
- Prepare six replicates of blank matrix from at least six different sources.
- Process each blank matrix through the entire sample preparation procedure.
- Spike posaconazole at three quality control levels (low, medium, high) into the processed blank extracts.
- Prepare corresponding reference standards in reconstitution solution at identical concentrations.
- Analyze all samples using the optimized HPLC-DAD method [1].
- Calculate matrix effect (ME) using the formula: ME (%) = (Mean peak area of spiked post-extraction sample / Mean peak area of reference standard) × 100
A ME value of 100% indicates no matrix effect, <100% indicates suppression, and >100% indicates enhancement. Acceptance criteria typically require ME values between 85-115% with CV ≤15% [47] [48].
Mitigation Strategies for Matrix Effects
Sample Preparation Optimization
Effective sample cleanup remains the most direct approach to minimize matrix effects. For posaconazole quantification in biological samples, several extraction techniques have demonstrated efficacy.
Liquid-Liquid Extraction (LLE) Protocol:
- Aliquot 500 μL of plasma sample into a glass tube.
- Add internal standard (e.g, itraconazole at 10 μg/mL) [1].
- Alkalinize sample with 100 μL of 0.1M sodium carbonate (for urine samples, this step is essential) [48].
- Add 3 mL of n-octanol (log P = 3.0) as extraction solvent [48].
- Vortex mix vigorously for 60 seconds.
- Centrifuge at 4000 × g for 10 minutes.
- Transfer organic layer to a clean tube.
- Evaporate to dryness under nitrogen stream at 40°C.
- Reconstitute residue with 200 μL of mobile phase.
- Vortex mix for 30 seconds and transfer to autosampler vials.
This LLE protocol demonstrated excellent extraction efficiency for clozapine and metabolites in plasma and urine, with minimal matrix interference [48]. For posaconazole specifically, LLE with n-octanol provided sufficient cleanup for accurate quantification in suspension dosage forms [1].
Alternative: Supramolecular Solvent (SUPRAS) Extraction: For more challenging matrices, SUPRAS formation offers an innovative extraction approach:
- Combine 1 mL of plasma with 1 mL of n-octanol and 0.5 mL of THF.
- Vortex mix for 60 seconds and centrifuge at 4000 × g for 10 minutes.
- Collect the coacervate phase (typically 100-200 μL) for analysis.
- Note: Injection volume limitations apply (10 μL for THF-based SUPRAS) [48].
Chromatographic Optimization
Strategic chromatographic method development can significantly reduce matrix interference by separating the analyte from co-eluting matrix components.
UHPLC-UV Method for Posaconazole:
- Column: Kinetex C18 (2.1 × 50 mm, 1.3 μm) [1]
- Mobile Phase: Acetonitrile:15 mM potassium dihydrogen phosphate (45:55, v/v) [1]
- Flow Rate: 0.4 mL/min [1]
- Temperature: 40°C [1]
- Injection Volume: 5 μL [1]
- Detection: 262 nm [1]
- Run Time: 3 minutes [1]
HPLC-DAD Method for Posaconazole:
- Column: Zorbax SB-C18 (4.6 × 250 mm, 5 μm) [1]
- Mobile Phase: Gradient elution from acetonitrile:15 mM potassium dihydrogen phosphate (30:70 to 80:20) over 7 minutes [1]
- Flow Rate: 1.5 mL/min [1]
- Temperature: 25°C [1]
- Injection Volume: 20-50 μL [1]
- Detection: 262 nm [1]
- Run Time: 11 minutes [1]
The UHPLC-UV method demonstrated superior chromatographic separation with shorter analysis time, potentially reducing matrix interference through improved peak efficiency [1].
Internal Standard Calibration
The internal standard method represents one of the most effective approaches to compensate for residual matrix effects, provided an appropriate internal standard is selected.
Selection Criteria:
- Structural analog to posaconazole (e.g., itraconazole) [1]
- Similar chromatographic behavior and extraction efficiency
- No interference from endogenous matrix components
- Chemical stability throughout analysis
Implementation Protocol:
- Add a fixed concentration of internal standard (e.g., 10 μg/mL itraconazole) to all calibration standards, quality controls, and study samples [1].
- Prepare calibration curves using peak area ratio (analyte/internal standard) versus concentration.
- Calculate sample concentrations using the regression equation derived from the calibration curve.
This approach effectively corrects for variability in sample preparation and injection volume, while partially compensating for matrix effects [49].
Table 2: Analytical Performance of Posaconazole Methods with Matrix Effect Mitigation
| Parameter | HPLC-DAD Method [1] | UHPLC-UV Method [1] | Acceptance Criteria |
|---|---|---|---|
| Linearity Range (μg/mL) | 5-50 | 5-50 | R² > 0.999 |
| LOD (μg/mL) | 0.82 | 1.04 | - |
| LOQ (μg/mL) | 2.73 | 3.16 | - |
| Intra-day Precision (%CV) | <3% | <3% | <15% |
| Inter-day Precision (%CV) | <3% | <3% | <15% |
| Matrix Effect (%) | 85-115* | 85-115* | 85-115% |
| Extraction Recovery (%) | >90* | >90* | >85% |
*Theorized values based on comparable methodologies described in [1] [48].
Integrated Workflow for Matrix Effect Mitigation
The following workflow integrates multiple strategies for comprehensive matrix effect management in posaconazole quantification:
Research Reagent Solutions
Table 3: Essential Research Reagents for Matrix Effect Mitigation
| Reagent/ Material | Function/Purpose | Example Specifications |
|---|---|---|
| n-Octanol [48] | Liquid-liquid extraction solvent; effectively extracts analytes while minimizing phospholipid co-extraction | Log P = 3.0; HPLC grade |
| C18 Chromatographic Column [1] [21] | Stationary phase for reversed-phase separation; different dimensions and particle sizes available | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) or Kinetex C18 (2.1 × 50 mm, 1.3 μm) |
| Potassium Dihydrogen Phosphate [1] [21] | Mobile phase buffer component; maintains consistent pH for reproducible retention times | 15 mM in water, HPLC grade |
| Acetonitrile [1] [21] | Organic mobile phase component; strong elution strength for posaconazole | HPLC grade, low UV absorbance |
| Itraconazole [1] | Internal standard; corrects for variability in extraction and analysis | Pharmaceutical grade, >99% purity |
| Solid Phase Extraction Cartridges [47] | Alternative sample clean-up; selective retention of analytes vs. matrix interferences | C18 or mixed-mode sorbents |
Effective mitigation of matrix effects is essential for reliable posaconazole quantification in biological samples using HPLC-DAD and UHPLC-UV methodologies. A systematic approach combining optimized sample preparation, chromatographic separation, and appropriate internal standardization provides a robust framework for managing matrix interference. The protocols outlined herein enable researchers to achieve accurate, precise, and reproducible results essential for therapeutic drug monitoring and pharmacokinetic studies of posaconazole.
Achieving Green and Sustainable Chemistry Goals in HPLC Method Development
The integration of Green Analytical Chemistry (GAC) principles into high-performance liquid chromatography (HPLC) method development represents a critical evolution in pharmaceutical analysis, particularly for antifungal drugs like posaconazole. Traditional HPLC methods often rely on hazardous organic solvents and generate significant waste, creating environmental concerns and increased disposal costs [50]. The global analytical community is now prioritizing sustainability through reduced solvent consumption, minimized energy usage, and safer chemicals [51].
This application note explores the implementation of green chemistry principles specifically for posaconazole quantification, comparing conventional HPLC-DAD with advanced UHPLC-UV techniques. Posaconazole, a broad-spectrum triazole antifungal agent used for invasive fungal infections in immunocompromised patients, requires precise quantification for therapeutic drug monitoring and quality control [1]. By applying green metrics and sustainable methodologies, researchers can maintain analytical performance while significantly reducing environmental impact, aligning with the United Nations Sustainable Development Goals for responsible consumption and production [52].
Green Chemistry Principles in HPLC Method Development
Core Concepts and Frameworks
Green Analytical Chemistry is built upon twelve principles that emphasize waste prevention, safer solvents, energy efficiency, and real-time analysis [50]. These principles have evolved into comprehensive frameworks including:
- White Analytical Chemistry (WAC): An integrated approach that balances environmental impact (green), analytical performance (red), and practical and economic feasibility (blue) [50]. A method is considered sustainable when these three pillars are balanced, resulting in an overall "white" appearance when scored.
- Circular Analytical Chemistry (CAC): Focuses on minimizing waste and keeping materials in use through recycling and recovery processes [51].
- Analytical Quality by Design (AQbD): A systematic approach to method development that emphasizes risk assessment and design of experiments to optimize methods for both performance and sustainability [53].
The transition from classical HPLC to greener approaches involves multiple strategies, including solvent replacement, method miniaturization, reduced analysis time, and improved energy efficiency [50].
Greenness Assessment Tools
Several metric tools have been developed to quantitatively evaluate the environmental impact of analytical methods:
- AGREE (Analytical GREEnness Metric): Provides a comprehensive score from 0-1 based on twelve GAC principles [54].
- GAPI (Green Analytical Procedure Index): A pictogram that assesses method greenness across multiple parameters [53].
- BAGI (Blue Applicability Grade Index): Evaluates method practicality and cost-effectiveness [55].
- AGSA (Analytical Green Star Area): A newer tool that provides a visual representation of method greenness [52].
These tools help researchers objectively compare methods and identify areas for improvement in sustainability performance.
Comparative Analysis: HPLC-DAD vs. UHPLC-UV for Posaconazole Quantification
Method Parameters and Performance
A direct comparison of HPLC-DAD and UHPLC-UV methods for posaconazole quantification reveals significant differences in environmental impact and efficiency [1].
Table 1: Comparison of HPLC-DAD and UHPLC-UV Methods for Posaconazole Quantification
| Parameter | HPLC-DAD Method | UHPLC-UV Method |
|---|---|---|
| Stationary Phase | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) | Kinetex-C18 (2.1 × 50 mm, 1.3 μm) |
| Mobile Phase | Gradient: Acetonitrile:15 mM potassium dihydrogen orthophosphate (30:70 to 80:20) | Isocratic: Acetonitrile:15 mM potassium dihydrogen orthophosphate (45:55) |
| Flow Rate | 1.5 mL/min | 0.4 mL/min |
| Injection Volume | 20-50 μL | 5 μL |
| Run Time | 11 minutes | 3 minutes |
| Retention Time | Not specified | ~8.2 minutes [3] |
| Linear Range | 5-50 μg/mL | 5-50 μg/mL |
| LOD/LOQ | 0.82/2.73 μg/mL | 1.04/3.16 μg/mL |
| Solvent Consumption per Run | ~16.5 mL | ~1.2 mL |
| Theoretical Plates | Not specified | >2000 [3] |
Environmental and Practical Implications
The UHPLC-UV method demonstrates superior sustainability characteristics compared to conventional HPLC-DAD. The reduced column dimensions (2.1 × 50 mm vs. 4.6 × 250 mm) and smaller particle size (1.3 μm vs. 5 μm) contribute to faster analysis and lower solvent consumption [1]. The isocratic elution in UHPLC further simplifies method development and reduces equilibration time between runs.
The environmental benefits of UHPLC are substantial:
- Solvent consumption reduction: Approximately 93% less mobile phase used per analysis
- Analysis time reduction: 73% faster analysis enabling higher throughput
- Waste generation: Significant decrease in hazardous waste requiring disposal
These improvements align with multiple GAC principles, including prevention of waste, safer solvents, and design for energy efficiency [50]. The shorter run time (3 minutes vs. 11 minutes) also reduces energy consumption, contributing to a lower carbon footprint for the analytical laboratory.
Sustainable Method Development Protocols
Analytical Quality by Design (AQbD) Workflow
The AQbD approach provides a systematic framework for developing robust, sustainable HPLC methods [53]. The workflow integrates quality assessment with environmental considerations throughout method development.
Diagram 1: AQbD Method Development Workflow. The systematic approach ensures both method robustness and sustainability considerations are integrated throughout development.
Defining the Analytical Target Profile (ATP) and Critical Quality Attributes (CQAs)
The ATP for posaconazole quantification should specify the required sensitivity (LOD < 1 μg/mL), linear range (5-50 μg/mL), precision (CV% < 2%), and run time (< 5 minutes for green objectives) [1]. Key CQAs include resolution from related compounds, peak symmetry, retention time, and theoretical plates.
Risk Assessment and Design of Experiments (DoE)
Critical method parameters (CMPs) requiring optimization include:
- Mobile phase composition and pH
- Column temperature
- Flow rate
- Gradient profile
A fractional factorial design can efficiently screen these parameters with minimal experimental runs, reducing solvent consumption during method development [3]. For posaconazole, factors such as organic phase percentage (58%), methanol in organic phase (6%), mobile phase pH (7), and column temperature (39°C) have been identified as significant [3].
Green Solvent Selection and Replacement
Solvent selection represents the most significant opportunity for improving HPLC method sustainability [50].
Table 2: Greenness Assessment of Common HPLC Solvents
| Solvent | Environmental Impact | Health Impact | Safety Impact | Green Alternative |
|---|---|---|---|---|
| Acetonitrile | High volatility, toxic to aquatic life | Respiratory irritant | Flammable | Ethanol-water mixtures |
| Methanol | High volatility, slow biodegradation | Systemic toxin | Flammable | Isopropanol |
| n-Hexane | Ozone formation, persistent | Neurotoxic | Highly flammable | Heptane (less toxic) |
| Chloroform | Ozone depletion, water contaminant | Carcinogenic | Forms phosgene | Ethyl acetate |
Solvent Replacement Protocol
- Evaluate solvent properties: Assess environmental, health, and safety (EHS) parameters using CHEM21 or ACS GCI solvent selection guides [50].
- Test chromatographic compatibility: Ensure alternative solvents maintain resolution and peak symmetry. Ethanol-water mixtures often successfully replace acetonitrile-water systems with method adjustments [52].
- Optimize method parameters: Adjust flow rate, gradient profile, and column temperature to compensate for changes in solvent strength and viscosity.
- Validate method performance: Confirm that the green method meets all ATP requirements including specificity, linearity, accuracy, and precision.
For posaconazole analysis, the mobile phase can be modified by replacing acetonitrile with ethanol-based systems or employing micellar liquid chromatography with surfactants like Brij-35 to eliminate organic solvents entirely [55].
Method Transfer and Scaling for Sustainability
Transferring methods from HPLC to UHPLC platforms significantly enhances sustainability:
- Column selection: Choose sub-2μm particles in shorter columns (50-100 mm) for improved efficiency [1].
- Flow rate adjustment: Calculate appropriate flow rates based on column dimension changes while considering pressure limitations.
- Gradient transfer: Apply scaling rules to maintain equivalent chromatographic separation [50].
- System suitability: Verify performance meets acceptance criteria with the transferred method.
The method transfer from HPLC to UHPLC for posaconazole reduces solvent consumption from ~16.5 mL to ~1.2 mL per analysis while maintaining linearity (r² > 0.999) and precision (CV% < 2%) [1].
White Analytical Chemistry Framework
The White Analytical Chemistry (WAC) framework provides a balanced approach to evaluate method sustainability across three dimensions: analytical, environmental, and practical [50].
Diagram 2: White Analytical Chemistry Framework. Sustainable methods balance analytical performance, environmental impact, and practical feasibility.
Integrated Assessment Protocol
To evaluate posaconazole quantification methods using the WAC framework:
- Analytical performance (Red): Assess specificity, accuracy, precision, linearity, and robustness according to ICH Q2(R2) guidelines. The method should demonstrate resolution from potential impurities and degradation products [52].
- Environmental impact (Green): Calculate greenness scores using AGREE, GAPI, or Analytical Eco-Scale tools. Target an AGREE score >0.7 for acceptable greenness [55].
- Practical and economic feasibility (Blue): Evaluate method cost, time requirements, equipment needs, and transferability to quality control environments.
Methods achieving balanced scores across all three dimensions are considered "white" and represent the most sustainable approach for routine implementation.
Essential Research Reagent Solutions
Table 3: Key Reagents and Materials for Sustainable Posaconazole HPLC Analysis
| Reagent/Material | Function | Green Alternative | Application Notes |
|---|---|---|---|
| Acetonitrile (HPLC grade) | Organic modifier in mobile phase | Ethanol (HPLC grade) | Higher viscosity requires method adjustment; biodegradable |
| Methanol (HPLC grade) | Organic solvent for sample preparation | Isopropanol | Less toxic alternative for extraction |
| Potassium dihydrogen phosphate | Buffer component in mobile phase | Ammonium formate | More volatile, MS-compatible |
| C18 column (250 × 4.6 mm, 5μm) | Stationary phase for separation | C18 column (50 × 2.1 mm, 1.3μm) | Reduced solvent consumption, faster analysis |
| Dichloromethane | Extraction solvent | Ethyl acetate | Less toxic, biodegradable |
| Phosphoric acid | Mobile phase pH adjustment | Formic acid | More volatile, MS-compatible |
The integration of green and sustainable chemistry principles into HPLC method development for posaconazole quantification provides significant environmental benefits without compromising analytical performance. The comparison between HPLC-DAD and UHPLC-UV methods demonstrates that modern approaches can reduce solvent consumption by over 90% and analysis time by nearly 75% while maintaining regulatory compliance [1].
The implementation of AQbD frameworks, green solvent replacements, and comprehensive assessment tools like White Analytical Chemistry enables researchers to develop methods that align with global sustainability goals. As regulatory agencies increasingly emphasize environmental considerations, these sustainable practices will become standard requirements in pharmaceutical analysis [51] [50].
For posaconazole analysis specifically, the transition to UHPLC-UV with isocratic elution represents an optimal balance of analytical performance, practical implementation, and reduced environmental impact, serving as a model for sustainable method development across pharmaceutical compounds.
Validation According to ICH Guidelines and Direct Method Comparison
Within the context of a broader thesis on the quantitation of posaconazole, the establishment of robust analytical methods is paramount. This application note details the core validation parameters—linearity, range, precision, and accuracy—for the quantification of posaconazole using High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) and Ultra-High-Performance Liquid Chromatography with Ultraviolet Detection (UHPLC-UV). These parameters, validated per International Conference on Harmonisation (ICH) guidelines, ensure that the methods are suitable for their intended purpose, whether for quality control of pharmaceutical dosage forms or for supporting pharmacokinetic studies [1] [2].
The following tables summarize key validation data from published methods for posaconazole quantification, illustrating the performance of different chromatographic approaches.
Table 1: Validation Parameters for Posaconazole in Formulations
| Parameter | HPLC-DAD Method [1] | UHPLC-UV Method [1] | HPLC-UV Method (Bulk & Tablet) [2] |
|---|---|---|---|
| Linearity Range | 5–50 μg/mL | 5–50 μg/mL | 2–20 μg/mL |
| Correlation (r²) | > 0.999 | > 0.999 | Not Specified |
| Precision (CV%) | < 3% | < 3% | < 1% (Intra- & Inter-day) |
| Accuracy (% Recovery) | Not Specified | Not Specified | 99.01% (Bulk), 99.05% (Tablet) |
| Limit of Detection (LOD) | 0.82 μg/mL | 1.04 μg/mL | Calculated per ICH |
| Limit of Quantitation (LOQ) | 2.73 μg/mL | 3.16 μg/mL | Calculated per ICH |
Table 2: Validation Parameters for Posaconazole in Biological Matrices
| Parameter | HPLC-DAD (Rat Plasma) [4] | HPLC-UV (Rat Plasma) [3] | HPLC-UV (Spiked Plasma) [46] |
|---|---|---|---|
| Linearity Range | 50–5000 ng/mL | 50–2000 ng/mL | 0.25–32 μg/mL |
| Precision (CV%) | ≤ 18% | Not Specified | < 2% |
| Accuracy (% Recovery) | Determined via extraction efficiency | Not Specified | 97.7 – 101.12% |
| Limit of Quantitation (LOQ) | 50 ng/mL | 50 ng/mL | Not Specified |
Experimental Protocols
Protocol for HPLC-DAD and UHPLC-UV Analysis of Suspension Dosage Form
This protocol is adapted from the comparative study of HPLC-DAD and UHPLC-UV assays [1].
3.1.1 Materials and Reagents
- Analytical Standards: Posaconazole bulk powder, Itraconazole (Internal Standard, IS).
- Solvents: HPLC-grade methanol and acetonitrile.
- Buffer: 15 mM Potassium dihydrogen orthophosphate.
- Sample: Posaconazole oral suspension (e.g., Noxafil 40 mg/mL).
3.1.2 Chromatographic Conditions
- HPLC-DAD Method:
- Column: Zorbax SB-C18 (4.6 × 250 mm, 5 μm).
- Mobile Phase: Gradient elution from Acetonitrile:15 mM phosphate buffer (30:70) to (80:20) over 7 minutes.
- Flow Rate: 1.5 mL/min.
- Detection: 262 nm.
- Injection Volume: 20-50 μL.
- Run Time: 11 minutes.
- UHPLC-UV Method:
- Column: Kinetex-C18 (2.1 × 50 mm, 1.3 μm).
- Mobile Phase: Isocratic Acetonitrile:15 mM phosphate buffer (45:55).
- Flow Rate: 0.4 mL/min.
- Detection: 262 nm.
- Injection Volume: 5 μL.
- Run Time: 3 minutes.
- HPLC-DAD Method:
3.1.3 Sample Preparation
- Dilute 0.1 mL of oral suspension to 10 mL with methanol (Solution S1).
- Add a fixed volume of IS working solution (10 μg/mL) to 0.1 mL of S1.
- Dilute to a final volume of 1 mL with methanol (Solution S2).
- Vortex mix for 10 seconds before injection.
3.1.4 Validation Workflow The procedure for establishing and validating the analytical method is systematically outlined below.
Figure 1: Workflow for Analytical Method Validation
Protocol for HPLC-UV Analysis in Low-Volume Plasma
This protocol is adapted from a recent study optimizing PCZ analysis for pharmacokinetic studies [3].
3.2.1 Materials and Reagents
- Analytical Standards: Posaconazole, Diazepam (Internal Standard).
- Solvents: HPLC-grade acetonitrile, methanol, and tertiary butyl methyl ether (TBME).
- Other Reagents: Sodium chloride, Orthophosphoric acid.
3.2.2 Chromatographic Conditions (Optimized)
- Column: PerfectSil Target C8 (250 × 4.6 mm, 5 μm).
- Mobile Phase: Combination of ACN and MeOH as organic phase with aqueous buffer (optimized via experimental design).
- Flow Rate: 1.2 mL/min.
- Detection: 262 nm.
- Column Temperature: 39 °C.
- Injection Volume: 100 μL.
3.2.3 Sample Preparation (Liquid-Liquid Extraction)
- To a 100 μL aliquot of spiked plasma, add the internal standard.
- Add 500 μL of TBME (extraction solvent) and 10% w/v sodium chloride solution.
- Adjust the plasma pH to 11 using a suitable base.
- Vortex mix for 10 minutes for extraction.
- Centrifuge at high speed for 1 minute.
- Transfer the organic layer and evaporate to dryness.
- Reconstitute the residue with the mobile phase and inject.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for Posaconazole Quantification
| Item | Function / Application | Example from Literature |
|---|---|---|
| C18 Columns | Reversed-phase separation of analytes. | Zorbax SB-C18 (5μm), Kinetex-C18 (1.3μm), Hyperclone C18 (5μm) [1] [2] |
| Acetonitrile & Methanol | Organic modifiers in the mobile phase for eluting analytes. | HPLC-grade used in mobile phase compositions [1] [3] [2] |
| Phosphate Buffer | Aqueous component of mobile phase; controls pH and ionic strength. | 15 mM Potassium dihydrogen orthophosphate, pH-adjusted [1] [4] |
| Posaconazole Reference Standard | Primary standard for preparing calibration curves and quality control samples. | Obtained from commercial suppliers (e.g., Selleckchem, BrightGene Co.) [1] [3] |
| Internal Standards | Corrects for variability in sample preparation and injection. | Itraconazole, Diazepam [1] [3] [4] |
| Protein Precipitation Solvents | (For Plasma) Deproteinizes plasma samples for cleaner analysis. | Acetonitrile or Methanol [3] [46] |
| Liquid-Liquid Extraction Solvents | (For Plasma) Extracts analyte from plasma matrix. | Tertiary butyl methyl ether (TBME), Diethyl ether [3] [4] |
Critical Discussion
The choice between HPLC-DAD and UHPLC-UV involves a trade-off between analysis time, solvent consumption, and sensitivity. The UHPLC-UV method demonstrates clear superiority in speed (3 min vs. 11 min) and reduced solvent usage, making it advantageous for high-throughput quality control labs [1]. However, the HPLC-DAD method showed a marginally better LOD and LOQ in one study [1]. For bioanalytical applications, the trend is toward methods that use low plasma volumes (100-200 μL) and achieve low LOQs (50 ng/mL), which is crucial for preclinical pharmacokinetic studies in small animals where blood volume is limited [3] [4]. The integration of advanced tools like experimental design (DoE) and machine learning (ML) models, such as Artificial Neural Networks (ANN) combined with Genetic Algorithms (GA), represents a modern, efficient approach to optimizing complex chromatographic and extraction parameters, ensuring robust method performance [3].
The establishment of validation parameters confirms that both HPLC-DAD and UHPLC-UV are highly suitable for the accurate and precise quantitation of posaconazole. The decision on which platform to use should be guided by the specific application, required throughput, and available resources. The detailed protocols and comparative data provided herein serve as a robust foundation for researchers and drug development professionals to implement these methods for quality control and therapeutic drug monitoring of posaconazole.
Assaying Specificity, Robustness, and System Suitability
The quantitative analysis of posaconazole (PSZ), a broad-spectrum triazole antifungal agent, is critical for ensuring its efficacy and safety in clinical practice, particularly for immunocompromised patients. High-Performance Liquid Chromatography (HPLC) and Ultra-High-Performance Liquid Chromatography (UHPLC) represent two foundational technological approaches for this task, each with distinct advantages and limitations. Specificity, robustness, and system suitability are three pillars of analytical method validation that ensure the reliability of results generated by these chromatographic systems. This document details application notes and protocols for evaluating these parameters, framed within a broader thesis investigating the quantitation of posaconazole using HPLC with Diode Array Detection (DAD) versus UHPLC with Ultraviolet Detection (UV). The protocols herein are designed to provide researchers, scientists, and drug development professionals with clear methodologies for validating their analytical procedures according to international guidelines [4] [1] [21].
Critical Comparison of HPLC-DAD and UHPLC-UV for Posaconazole Analysis
The fundamental difference between HPLC and UHPLC lies in the operating pressure and particle size of the column packing material. UHPLC systems operate at significantly higher pressures and utilize columns with smaller particles (typically below 2 μm), which confers advantages in speed, resolution, and solvent consumption [1]. A direct comparative study of PSZ quantification demonstrated that an HPLC-DAD assay had a run time of 11 minutes, while a UHPLC-UV assay achieved a similar separation in just 3 minutes [1] [45]. Both methods exhibited excellent linearity (r² > 0.999) and comparable precision (CV% < 3%) [1]. The following table summarizes key performance metrics from validated methods for posaconazole quantification.
Table 1: Comparison of Chromatographic Methods for Posaconazole Quantification
| Method Parameter | HPLC-DAD (Bulk/Suspension) [1] | UHPLC-UV (Bulk/Suspension) [1] | HPLC-DAD (Rat Plasma) [4] | HPLC-UV (Human Serum) [56] |
|---|---|---|---|---|
| Column | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) | Kinetex-C18 (2.1 × 50 mm, 1.3 μm) | HC-C18 (4.6 × 250 mm, 5 μm) | Not Specified |
| Mobile Phase | Gradient: ACN / 15 mM KH₂PO₄ | Isocratic: ACN / 15 mM KH₂PO₄ (45:55) | Gradient: ACN / 0.015 M KH₂PO₄ | Not Specified |
| Flow Rate (mL/min) | 1.5 | 0.4 | 1.5 | Not Specified |
| Run Time (min) | 11 | 3 | 11 | 11 |
| Detection Wavelength (nm) | 262 | 262 | PSZ: 262; VCR: 220 | 262 |
| Linearity Range | 5–50 μg/mL | 5–50 μg/mL | 50–5000 ng/mL | 0.125–16 μg/mL |
| Limit of Quantification (LOQ) | 2.73 μg/mL | 3.16 μg/mL | 50 ng/mL | 0.125 μg/mL |
Experimental Protocols
Reagent Solutions and Materials
The successful execution of these analytical methods requires specific, high-quality materials. The following table lists essential research reagents and their functions.
Table 2: Essential Research Reagent Solutions for Posaconazole Analysis
| Reagent/Material | Function/Application | Specifications/Examples |
|---|---|---|
| Posaconazole Reference Standard | Primary analyte for quantification and calibration curve preparation. | Purity >99% (e.g., Selleckchem) [4] [1]. |
| Itraconazole (ITZ) | Internal Standard (IS) for HPLC-DAD/UHPLC-UV assays to correct for procedural variability [4] [1]. | Purity >99%. |
| Diazepam | Internal Standard for alternative HPLC-UV methods in biological matrices [3]. | Purity >99%. |
| HPLC-Grade Acetonitrile & Methanol | Organic modifiers in the mobile phase and for preparation of stock/standard solutions. | Fisher Scientific UK Ltd. [4] [1]. |
| Potassium Dihydrogen Orthophosphate (KH₂PO₄) | Component of the aqueous buffer in the mobile phase to control pH and improve separation. | Analytical grade (e.g., Riedel-de-Haën) [4] [1]. |
| Diethyl Ether / Tertiary Butyl Methyl Ether (TBME) | Organic solvents for liquid-liquid extraction of posaconazole from plasma samples [4] [3]. | HPLC or analytical grade. |
| C18 Reversed-Phase Columns | Stationary phase for chromatographic separation. | e.g., Zorbax SB-C18, Phenomenex Hyperclone C18 [4] [1] [21]. |
Protocol for Specificity and Selectivity Testing
Principle: Specificity is the ability of the method to accurately measure the analyte (posaconazole) in the presence of other components such as excipients, degradation products, metabolites, or co-administered drugs [21].
Procedure:
- Preparation of Solutions:
- Standard Solution: Prepare a solution of posaconazole at a concentration within the linear range (e.g., 20 μg/mL for bulk, 500 ng/mL for plasma) in the appropriate solvent [21].
- Blank Matrix: Process the sample matrix (e.g., mobile phase, placebo formulation, or drug-free plasma) without the analyte.
- Spiked Matrix: Process the blank matrix spiked with posaconazole and the internal standard (e.g., itraconazole).
- Forced Degradation Samples: Expose posaconazole solutions to stress conditions (acid, base, oxidation, heat, light) and analyze the degraded samples [1].
- Co-administered Drug Solution: In drug-interaction studies, prepare solutions of relevant drugs (e.g., Vincristine) [4].
Chromatographic Analysis:
- Inject the prepared solutions into the HPLC or UHPLC system using the conditions outlined in Table 1.
- For HPLC-DAD, utilize the diode array detector to obtain peak purity spectra by scanning from 200 nm to 400 nm.
Data Analysis and Acceptance Criteria:
- The chromatogram of the blank matrix should show no interfering peaks at the retention times of posaconazole and the internal standard [57].
- The peak purity index for posaconazole, as determined by the DAD, should be above a pre-defined threshold (e.g., 999), confirming a homogeneous peak without co-eluting impurities.
- In the case of Vincristine and Posaconazole co-analysis, the method must baseline-resolve both analytes and the IS, as demonstrated in a validated assay where all three compounds were separated within 11 minutes [4].
Protocol for Robustness Testing
Principle: Robustness evaluates the reliability of an analytical method when small, deliberate variations are made to its operational parameters. It is an indicator of the method's suitability for routine use.
Procedure:
- Experimental Design:
- A fractional 2-level factorial design is highly efficient for evaluating multiple factors simultaneously [3]. Key chromatographic factors to vary include:
- A: Total organic phase composition in mobile phase (± 2%)
- B: Methanol ratio in the organic phase (± 2%)
- C: pH of the aqueous buffer (± 0.1 units)
- D: Column temperature (± 2°C)
- E: Flow rate (± 0.1 mL/min)
- A fractional 2-level factorial design is highly efficient for evaluating multiple factors simultaneously [3]. Key chromatographic factors to vary include:
Execution:
- Prepare a standard solution of posaconazole at a mid-level concentration (e.g., 20 μg/mL).
- Analyze this solution under the nominal (central) conditions and at all combinations of the high and low levels for the selected factors.
- Run the experiments in a randomized order to minimize bias.
Data Analysis and Acceptance Criteria:
- For each experimental run, record the retention time (RT), peak area, tailing factor, and theoretical plates for posaconazole.
- The method is considered robust if the system suitability parameters (see Section 3.4) remain within acceptable limits across all variations.
- Analysis of Variance (ANOVA) can be used to identify factors that have a statistically significant (p-value < 0.05) effect on the responses [3].
Protocol for System Suitability Testing
Principle: System suitability tests (SST) are an integral part of chromatographic methods and verify that the total system—instrument, reagents, and column—is performing adequately at the time of analysis.
Procedure:
- Preparation: Prepare a standard solution of posaconazole and internal standard at a specified concentration (e.g., 20 μg/mL) in the mobile phase or matrix.
Chromatographic Analysis: Inject this solution in six replicates.
Data Analysis and Acceptance Criteria:
- Calculate the following parameters from the resulting chromatograms:
- Theoretical Plates (N): A measure of column efficiency. Should be > 2000.
- Tailing Factor (T): A measure of peak symmetry. Should be ≤ 2.0.
- Relative Standard Deviation (RSD%) of Retention Time and Peak Area: A measure of repeatability. RSD for peak area of the six replicates should be ≤ 2.0% [21].
- Resolution (Rs): When analyzing a mixture, resolution from the closest eluting peak should be > 1.5 [4].
- Calculate the following parameters from the resulting chromatograms:
Diagram: System Suitability Testing Workflow
Application in a Pharmacokinetic Study
The validated HPLC-DAD method has been successfully applied in a preclinical drug-drug interaction study. In this protocol [4]:
- Two rats were orally dosed with 40 mg/kg PSZ, followed by an intravenous dose of 0.1 mg/kg Vincristine (VCR) 30 minutes later.
- Serial blood samples (a low volume of 200 μL or less was sufficient for the assay [4] [3]) were collected over 72 hours via retro-orbital sampling.
- Plasma was separated by centrifugation and stored at -20°C until analysis.
- Samples were processed using the liquid-liquid extraction procedure with diethyl ether, and the residues were reconstituted and analyzed via the described HPLC-DAD method.
- The assay simultaneously quantified PSZ and VCR concentrations at each time point from a single blood draw, facilitating the calculation of pharmacokinetic parameters like AUC and half-life, which were found to be comparable to literature values [4]. This demonstrates the method's practical utility in complex biological studies.
The rigorous assessment of specificity, robustness, and system suitability is paramount for developing reliable HPLC-DAD and UHPLC-UV methods for posaconazole quantitation. HPLC-DAD offers robust performance and the advantage of peak purity confirmation, which is crucial for specificity. UHPLC-UV provides superior speed and solvent economy, which is beneficial for high-throughput environments. The choice between the two should be guided by the specific application, available instrumentation, and required throughput. The protocols detailed in this document provide a clear roadmap for scientists to validate their analytical methods, ensuring the generation of accurate and reproducible data for quality control, therapeutic drug monitoring, and advanced pharmacokinetic research.
The quantitative analysis of posaconazole, a broad-spectrum triazole antifungal agent, is critical for pharmaceutical quality control and therapeutic drug monitoring [1] [21]. High-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC) represent two foundational technological approaches for such analyses, each with distinct operational characteristics [1] [58]. This application note provides a detailed head-to-head comparison of HPLC-Diode Array Detection (DAD) and UHPLC-Ultraviolet (UV) methodologies for posaconazole quantification, focusing specifically on analysis time, solvent consumption, and cost-efficiency within a research context. The data presented herein aims to equip researchers and drug development professionals with empirical evidence to inform platform selection based on specific laboratory requirements and constraints.
The fundamental technological difference between these platforms lies in the column packing material particle size and the resulting system pressure requirements. UHPLC utilizes columns packed with smaller particles (typically <2 μm), enabling higher efficiency separations but requiring instrumentation capable of withstanding significantly higher operating pressures [1] [59]. This core difference drives the observed variations in analysis speed, solvent usage, and operational economics.
Experimental Protocols
HPLC-DAD Method for Posaconazole Quantification
2.1.1 Materials and Reagents
- Posaconazole standard (e.g., Selleckchem) [1]
- Itraconazole internal standard [1]
- HPLC-grade methanol and acetonitrile (Fisher Scientific UK Ltd.) [1]
- Potassium dihydrogen orthophosphate (analytical grade, Riedel-de-Haën) [1]
- High purity distilled water [1]
- Zorbax SB-C18 column (4.6 × 250 mm, 5 μm particle size, Agilent Technologies) [1]
2.1.2 Instrumentation and Conditions
- Chromatography system: Agilent 1200 series with quaternary pump, vacuum degasser, and diode array detector (G1315 C/D and G1365 C/D) [1]
- Detection wavelength: 262 nm [1]
- Mobile phase: Gradient elution of acetonitrile:15 mM potassium dihydrogen orthophosphate (30:70 to 80:20, linear over 7 minutes) [1]
- Flow rate: 1.5 mL/min [1]
- Injection volume: 20-50 μL [1]
- Column temperature: 25°C [1]
- Run time: 11 minutes [1]
2.1.3 Sample Preparation
- Prepare stock solution of posaconazole at 100 μg/mL in methanol [1]
- Prepare working solutions through serial dilution with methanol to achieve calibration range of 5-50 μg/mL [1]
- Add internal standard (10 μg/mL itraconazole) to samples [1]
- For suspension formulations, dilute 0.1 mL of suspension to 10 mL with methanol, then further dilute with internal standard solution [1]
UHPLC-UV Method for Posaconazole Quantification
2.2.1 Materials and Reagents
- Posaconazole standard (e.g., Selleckchem) [1]
- Itraconazole internal standard [1]
- HPLC-grade methanol and acetonitrile [1]
- Potassium dihydrogen orthophosphate (analytical grade) [1]
- High purity distilled water [1]
- Kinetex-C18 column (2.1 × 50 mm, 1.3 μm particle size, Phenomenex) [1]
2.2.2 Instrumentation and Conditions
- Chromatography system: Agilent 1290 Infinity Binary Pump LC with UV detector [1]
- Detection wavelength: 262 nm [1]
- Mobile phase: Isocratic elution of acetonitrile:15 mM potassium dihydrogen orthophosphate (45:55) [1]
- Flow rate: 0.4 mL/min [1]
- Injection volume: 5 μL [1]
- Column temperature: 40°C [1]
- Run time: 3 minutes [1]
Method Validation
Both methods were validated according to International Conference on Harmonisation (ICH) guidelines, assessing linearity, precision, accuracy, limits of detection (LOD), and limits of quantitation (LOQ) [1] [21]. The validation procedures included:
- Linearity: Constructed calibration curves over concentration range of 5-50 μg/mL [1]
- Precision: Evaluated through intra-day and inter-day variations with coefficient of variation (CV%) <3% [1]
- Accuracy: Determined through recovery studies with percentage error of the mean <3% [1]
- Specificity: Confirmed absence of interference from excipients in pharmaceutical formulations [1]
Results and Discussion
Direct Performance Comparison
Table 1: Direct comparison of HPLC-DAD and UHPLC-UV methods for posaconazole analysis
| Parameter | HPLC-DAD | UHPLC-UV | Advantage Ratio |
|---|---|---|---|
| Analysis Time (per run) | 11 minutes [1] | 3 minutes [1] | UHPLC: 3.7x faster |
| Solvent Consumption (per run) | ~16.5 mL [1] | ~1.2 mL [1] | UHPLC: 13.8x less solvent |
| Injection Volume | 20-50 μL [1] | 5 μL [1] | UHPLC: 4-10x lower volume |
| Column Dimensions | 4.6 × 250 mm [1] | 2.1 × 50 mm [1] | UHPLC: Smaller column |
| Particle Size | 5 μm [1] | 1.3 μm [1] | UHPLC: Smaller particles |
| Limit of Detection | 0.82 μg/mL [1] | 1.04 μg/mL [1] | HPLC: Slightly more sensitive |
| Limit of Quantification | 2.73 μg/mL [1] | 3.16 μg/mL [1] | HPLC: Slightly better |
The experimental data reveals that UHPLC-UV demonstrates superior performance in analysis speed and solvent economy, while maintaining comparable analytical figures of merit regarding linearity, precision, and accuracy [1]. The significantly reduced solvent consumption with UHPLC-UV (approximately 14-fold less) translates to substantial cost savings in routine analysis, particularly when analyzing large sample batches. Furthermore, the reduced analysis time increases laboratory throughput potential by nearly four-fold [1].
Economic and Operational Implications
The economic advantages of UHPLC extend beyond direct solvent savings. Reduced solvent consumption also decreases waste disposal costs and environmental impact, aligning with green chemistry principles [58]. A separate study comparing HPLC and UHPLC methods for guanylhydrazone derivatives similarly reported that UHPLC provided "four times less solvent consumption" alongside faster analysis times [58].
However, the initial capital investment for UHPLC instrumentation typically exceeds that of conventional HPLC systems. Additionally, UHPLC columns generally carry a higher price point than their HPLC counterparts, though this cost differential has narrowed in recent years. The return on investment calculation must consider the projected sample volume, with high-throughput laboratories benefiting more rapidly from UHPLC adoption.
Analytical Performance Considerations
Despite the operational advantages of UHPLC, both techniques demonstrated excellent analytical performance for posaconazole quantification. Both methods exhibited linearity (r² > 0.999) across the validated concentration range, with precision (CV%) and accuracy (% error) meeting ICH validation criteria [1]. The slightly superior LOD and LOQ values observed with HPLC-DAD (0.82 and 2.73 μg/mL versus 1.04 and 3.16 μg/mL for UHPLC-UV) may be significant for applications requiring trace-level detection, though both methods adequately cover the therapeutic range of posaconazole [1].
The DAD detection in the HPLC method provides an additional capability for peak purity assessment and spectral confirmation, which can be valuable in method development and impurity profiling [60]. As noted in chromatographic literature, "While a UV detector captures data at a single, fixed wavelength, a DAD scans the entire UV-Vis spectrum, uncovering details that would otherwise remain hidden" [60]. This capability comes at a higher instrument cost but provides enhanced data integrity.
Research Reagent Solutions
Table 2: Essential materials and reagents for posaconazole analysis
| Item | Function/Role | HPLC-DAD Specification | UHPLC-UV Specification |
|---|---|---|---|
| Analytical Column | Stationary phase for compound separation | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) [1] | Kinetex-C18 (2.1 × 50 mm, 1.3 μm) [1] |
| Mobile Phase | Liquid medium carrying sample through column | Acetonitrile:15 mM potassium dihydrogen phosphate (gradient) [1] | Acetonitrile:15 mM potassium dihydrogen phosphate (isocratic) [1] |
| Internal Standard | Reference compound for quantification | Itraconazole [1] | Itraconazole [1] |
| Detection System | Analyte detection and quantification | Diode Array Detector (DAD) [1] | UV Detector [1] |
| Solvent Consumption | Mobile phase volume per analysis | ~16.5 mL/run [1] | ~1.2 mL/run [1] |
Method Selection Workflow
The following workflow diagram illustrates the decision-making process for selecting between HPLC-DAD and UHPLC-UV methods based on specific research requirements:
Method Selection Workflow
Both HPLC-DAD and UHPLC-UV methods provide reliable, validated approaches for posaconazole quantification in pharmaceutical formulations, with each platform offering distinct advantages. UHPLC-UV demonstrates superior performance in analysis speed (3.7x faster) and solvent economy (13.8x reduction), making it ideal for high-throughput laboratories and environments prioritizing operational efficiency and green chemistry principles [1] [58]. Conversely, HPLC-DAD remains a valuable approach for laboratories with budget constraints or those requiring the additional spectral information provided by diode array detection for method development and comprehensive peak purity assessment [60].
The selection between these platforms should be guided by specific research requirements, sample volume, available instrumentation, and budgetary considerations. Both methods successfully meet regulatory validation criteria and can be implemented confidently for quality control and research applications involving posaconazole quantification.
Evaluating Limits of Detection (LOD) and Quantification (LOQ) Across Platforms
The accurate quantification of active pharmaceutical ingredients, such as the antifungal drug posaconazole, is fundamental to pharmaceutical development and quality control. This process hinges on robust analytical methods characterized by well-defined sensitivity parameters, specifically the Limit of Detection (LOD) and Limit of Quantification (LOQ). The choice of analytical platform—whether High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) or Ultra-High-Performance Liquid Chromatography with Ultraviolet Detection (UHPLC-UV)—profoundly impacts these parameters and the overall efficiency of analysis [1]. This application note, framed within broader thesis research on posaconazole quantitation, provides a structured comparison of LOD and LOQ across these platforms. It summarizes critical quantitative data and delineates detailed experimental protocols to guide researchers and drug development professionals in selecting and validating appropriate methods for their specific applications.
Comparative Platform Performance: LOD and LOQ
The fundamental sensitivity of an analytical method is defined by its LOD (the lowest concentration of analyte that can be detected) and LOQ (the lowest concentration that can be quantified with acceptable precision and accuracy) [61]. A direct comparison of these parameters for posaconazole analysis reveals platform-specific advantages.
Table 1: Comparison of LOD and LOQ for Posaconazole on HPLC-DAD and UHPLC-UV Platforms
| Analytical Platform | Stationary Phase | LOD (µg/mL) | LOQ (µg/mL) | Linear Range (µg/mL) | Analysis Runtime | Key Advantages |
|---|---|---|---|---|---|---|
| HPLC-DAD [1] | Zorbax SB-C18 (4.6 × 250 mm, 5 μm) | 0.82 | 2.73 | 5–50 | 11 minutes | Robustness, wider availability |
| UHPLC-UV [1] | Kinetex-C18 (2.1 × 50 mm, 1.3 μm) | 1.04 | 3.16 | 5–50 | 3 minutes | Speed, superior separation, reduced solvent consumption |
| HPLC-UV (Plasma) [3] | PerfectSil Target C8 (250 × 4.6 mm, 5 μm) | - | 0.05* | 0.05–2.0* | 8.2 minutes | Optimized for low-volume plasma samples |
Note: Values denoted with * are in µg/mL, converted from the original 50 ng/mL for comparative purposes. The plasma method [3] demonstrates that with specialized optimization, even HPLC-UV can achieve very low LOQs in biological matrices.
The data in Table 1 illustrates a critical point: while UHPLC offers superior speed, the core LOD and LOQ values for posaconazole in formulation analysis can be comparable between the two platforms, with the HPLC-DAD method showing slightly better sensitivity in one direct comparison [1]. This highlights that factors such as column chemistry, detection settings, and sample preparation are as crucial as the platform choice itself. Furthermore, the methodology for calculating LOD and LOQ significantly influences the reported values. A study on carbamazepine and phenytoin analysis found that the signal-to-noise ratio (S/N) method yielded the lowest LOD/LOQ values, while the standard deviation of the response and slope method resulted in the highest values [61]. This underscores the necessity of clearly stating the calculation methodology in any method validation protocol.
Experimental Protocols for Method Comparison
To ensure the reliability of the LOD and LOQ data presented, a rigorous and validated experimental procedure must be followed. The following protocols are adapted from validated methods for posaconazole analysis [1] [21].
HPLC-DAD Protocol for Posaconazole Suspension
1. Reagent and Standard Preparation:
- Mobile Phase: Prepare 15 mM potassium dihydrogen orthophosphate (aqueous) and HPLC-grade acetonitrile (organic).
- Posaconazole Stock Solution (100 µg/mL): Accurately weigh 10 mg of posaconazole reference standard and dissolve in 100 mL of methanol.
- Internal Standard (IS) Solution (100 µg/mL): Dissolve 10 mg of itraconazole in 100 mL of methanol.
- Calibration Standards: Dilute the stock solution with methanol to prepare standards spanning the range of 5–50 µg/mL. Add a fixed concentration of IS (e.g., 10 µg/mL) to each standard.
2. Chromatographic Conditions:
- Column: Zorbax SB-C18 (4.6 × 250 mm, 5 μm)
- Elution Mode: Gradient. Initial composition of acetonitrile:15 mM phosphate (30:70), linearly changing to (80:20) over 7 minutes.
- Flow Rate: 1.5 mL/min
- Detection: DAD at 262 nm
- Injection Volume: 20-50 µL
- Column Temperature: 25°C
3. Sample Preparation (Oral Suspension):
- Dilute 0.1 mL of the suspension (40 mg/mL) to 10 mL with methanol (Solution S1).
- Add 0.1 mL of S1 and 10 µg/mL of IS to a microcentrifuge tube, then dilute to 1 mL with methanol (Solution S2).
- Vortex mix for 10 seconds and inject.
4. LOD/LOQ Determination:
- LOD and LOQ are determined empirically at signal-to-noise ratios of 3:1 and 10:1, respectively [1] [56]. Alternatively, they can be calculated from the standard deviation of the response and the slope of the calibration curve (SDR) [61].
UHPLC-UV Protocol for Posaconazole
1. Reagent and Standard Preparation:
- Prepare stock and standard solutions as described in the HPLC-DAD protocol.
2. Chromatographic Conditions:
- Column: Kinetex-C18 (2.1 × 50 mm, 1.3 μm)
- Elution Mode: Isocratic. Use a mobile phase of acetonitrile:15 mM potassium dihydrogen orthophosphate (45:55).
- Flow Rate: 0.4 mL/min
- Detection: UV at 262 nm
- Injection Volume: 5 µL
- Column Temperature: 40°C
3. Sample Preparation:
- Follow the same procedure as for HPLC-DAD.
4. LOD/LOQ Determination:
- Use the same principles as for HPLC-DAD (S/N ratio of 3:1 and 10:1).
Workflow and Platform Selection
The decision to use HPLC-DAD or UHPLC-UV depends on the application's specific requirements, including throughput, sensitivity, and available instrumentation. The following workflow diagrams the logical process for method selection and execution.
Diagram 1: Analytical method selection and validation workflow.
The Scientist's Toolkit: Essential Research Reagents and Materials
A successful analytical method relies on carefully selected reagents and materials. The following table lists key components used in the protocols for posaconazole analysis.
Table 2: Research Reagent Solutions for Posaconazole Quantitation
| Item | Function / Role | Example / Specification |
|---|---|---|
| Posaconazole Reference Standard | Primary analyte for quantification and calibration curve generation. | Pharmaceutical Secondary Standard (Selleckchem) [1]. |
| Itraconazole | Internal Standard (IS) to correct for procedural losses and injection variability. | Pharmaceutical grade (e.g., from Nifty Labs) [1]. |
| HPLC-Grade Acetonitrile & Methanol | Mobile phase component and solvent for stock/standard solutions. | Low UV absorbance, high purity (e.g., Fisher Scientific) [1] [21]. |
| Potassium Dihydrogen Orthophosphate | Buffer salt for aqueous mobile phase to control pH and improve peak shape. | Analytical grade (e.g., Riedel-de-Haën) [1]. |
| C18 Reverse-Phase Column | Stationary phase for chromatographic separation. | HPLC: Zorbax SB-C18 (5µm). UHPLC: Kinetex-C18 (1.3µm) [1]. |
| 0.22 µm Nylon Membrane Filter | Filtration of mobile phase and samples to remove particulates. | Essential for protecting UHPLC systems from blockages [21]. |
This application note demonstrates that both HPLC-DAD and UHPLC-UV platforms are capable of sensitive and reliable quantification of posaconazole, with distinct advantages for each. The choice between them involves a strategic trade-off between analysis speed and operational considerations. The provided protocols and data serve as a foundational guide for researchers. However, achieving optimal LOD and LOQ is not a one-size-fits-all process; it requires systematic optimization of every analytical parameter, from column selection and mobile phase composition to sample preparation and the very definition of sensitivity parameters [61] [3]. For thesis research or drug development work, a clear understanding of these factors ensures the development of a fit-for-purpose analytical method that guarantees the accurate quantification essential for patient safety and therapeutic efficacy.
This application note provides a structured framework for researchers and drug development professionals to select the optimal analytical technology for posaconazole quantification. Within the broader context of analytical method development for antifungal drugs, we evaluate High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) and Ultra-High-Performance Liquid Chromatography with UV Detection (UHPLC-UV). We present a practical decision matrix based on key performance criteria, supported by experimental data and detailed protocols, to guide selection between routine quality control (QC) and high-throughput analysis scenarios.
The accurate quantification of posaconazole, a broad-spectrum triazole antifungal drug, is critical for ensuring product quality, therapeutic efficacy, and patient safety in pharmaceutical development and manufacturing [2] [21]. HPLC-DAD and UHPLC-UV represent two advanced technological approaches with distinct advantages for different operational contexts.
HPLC-DAD offers the benefit of spectral confirmation for peak identity and purity, which is invaluable for method development and impurity profiling [60]. Conversely, UHPLC-UV, utilizing columns packed with smaller particles (<2 µm) and operating at higher pressures, provides significant enhancements in speed, resolution, and sensitivity [1]. This note establishes a systematic decision-making process, grounded in experimental data, to navigate the trade-offs between these technologies for specific application needs in the quantitation of posaconazole.
Comparative Instrumentation and Data
Key Technological Differences
The core differences between HPLC-DAD and UHPLC-UV systems extend beyond pressure capabilities to fundamental design and detection philosophies.
- Detection Capabilities: A DAD detector captures the full ultraviolet-visible spectrum for each data point, enabling peak purity assessment and method development by analyzing spectra across a peak. A UV detector, even a modern tunable one, typically monitors one or a few fixed wavelengths, offering lower noise levels which can be beneficial for achieving lower detection limits in certain applications [10] [60].
- System Performance: UHPLC systems are engineered for high-pressure operation (often exceeding 800 bar), with low-dispersion fluidic paths and high-speed detectors capable of fast data acquisition rates (40-60 Hz) to accurately capture narrow peaks. Traditional HPLC systems operate at lower pressures (typically up to 400 bar) and are the workhorses of many QC labs, with a wider range of available column phases [1] [10].
Quantitative Performance Comparison for Posaconazole
The following table summarizes performance data derived from validated methods for posaconazole analysis using both techniques.
Table 1: Comparative Analytical Performance of HPLC-DAD and UHPLC-UV for Posaconazole
| Performance Parameter | HPLC-DAD Method [1] | UHPLC-UV Method [1] | Green HPLC Method [62] |
|---|---|---|---|
| Run Time | 11 minutes | 3 minutes | ~3.4 minutes |
| Linearity Range | 5–50 µg/mL | 5–50 µg/mL | 1–25 µg/mL |
| Correlation Coefficient (r²) | >0.999 | >0.999 | 0.9999 |
| Limit of Detection (LOD) | 0.82 µg/mL | 1.04 µg/mL | Not Specified |
| Limit of Quantitation (LOQ) | 2.73 µg/mL | 3.16 µg/mL | Not Specified |
| Precision (% CV) | <3% | <3% | <1% |
| Mobile Phase Consumption/Run | ~16.5 mL | ~1.2 mL | ~1 mL |
The Scientist's Toolkit: Essential Research Reagent Solutions
The following table details key materials and reagents required for developing and executing the posaconazole quantification methods discussed.
Table 2: Essential Reagents and Materials for Posaconazole Analysis
| Item | Function / Role | Example Specifications / Notes |
|---|---|---|
| Posaconazole Reference Standard | Primary standard for calibration curve preparation; used to determine accuracy and method specificity. | Certified reference material of known high purity (>98%) [2]. |
| Chromatography Column (HPLC) | Stationary phase for analyte separation. | C18 column (e.g., Zorbax SB-C18, 4.6 × 250 mm, 5 µm) [1]. |
| Chromatography Column (UHPLC) | Stationary phase designed for high-pressure separations. | C18 column (e.g., Kinetex-C18, 2.1 × 50 mm, 1.3 µm) [1]. |
| HPLC-Grade Acetonitrile & Methanol | Organic modifiers in the mobile phase; critical for elution strength and selectivity. | Low UV cutoff, high purity to minimize baseline noise [2] [62]. |
| HPLC-Grade Water / Phosphate Buffer | Aqueous component of the mobile phase; buffer controls pH which impacts peak shape and retention. | 15 mM Potassium dihydrogen orthophosphate, pH-adjusted [1] [2]. |
| Internal Standard (e.g., Itraconazole) | Added to samples to correct for variability in injection volume and sample processing. | Should be structurally similar, stable, and not interfere with the analyte [1]. |
Experimental Protocols
Detailed Protocol: HPLC-DAD Method for Posaconazole
This protocol is adapted from established methods for the quantification of posaconazole in suspension dosage forms [1].
Instrumentation and Conditions:
- System: Agilent 1200 series or equivalent, equipped with a quaternary pump, autosampler, and DAD.
- Column: Zorbax SB-C18 (4.6 × 250 mm, 5 µm) or equivalent, maintained at 25°C.
- Mobile Phase: Gradient elution from Acetonitrile : 15 mM Potassium dihydrogen orthophosphate (30:70) to (80:20) linearly over 7 minutes.
- Flow Rate: 1.5 mL/min.
- Detection: DAD set at 262 nm; spectral acquisition from 200-400 nm for peak purity analysis.
- Injection Volume: 20-50 µL.
Sample Preparation:
- Stock Solution (100 µg/mL): Accurately weigh 10 mg of posaconazole reference standard into a 100 mL volumetric flask. Dissolve and make to volume with methanol.
- Calibration Standards: Prepare serial dilutions of the stock solution with methanol to cover the concentration range of 5-50 µg/mL.
- Sample Preparation (Oral Suspension): Transfer 0.1 mL of the suspension into a 10 mL volumetric flask. Dilute to volume with methanol and mix thoroughly (Solution S1). Pipette 0.1 mL of S1 into a 2.5 mL microcentrifuge tube, add 10 µg/mL of internal standard (e.g., Itraconazole), and dilute to 1 mL with methanol. Vortex mix for 10 seconds before injection.
Validation Steps:
- Specificity: Inject blank (diluent), standard, and sample preparations to confirm the absence of interfering peaks at the retention time of posaconazole. Use DAD spectrum to confirm peak homogeneity [2].
- Linearity: Inject each calibration standard in triplicate. Plot mean peak area (or area ratio to IS) against concentration. The correlation coefficient (r²) should be >0.999.
- Precision & Accuracy: Analyze QC samples at low, medium, and high concentrations within the linear range (e.g., 5, 20, 50 µg/mL) in six replicates. Intra-day and inter-day precision (CV%) should be <3%, and accuracy (% recovery) should be within 97-103% [1] [21].
Detailed Protocol: UHPLC-UV Method for Posaconazole
This protocol is optimized for speed and solvent economy, suitable for high-throughput environments [1].
Instrumentation and Conditions:
- System: Agilent 1290 Infinity or equivalent UHPLC system capable of operating at pressures up to 1200 bar.
- Column: Kinetex-C18 (2.1 × 50 mm, 1.3 µm) or equivalent, maintained at 40°C.
- Mobile Phase: Acetonitrile : 15 mM Potassium dihydrogen orthophosphate (45:55), delivered isocratically.
- Flow Rate: 0.4 mL/min.
- Detection: UV detector set at 262 nm.
- Injection Volume: 5 µL.
- Data Acquisition Rate: 40 Hz minimum to ensure accurate peak integration.
Sample Preparation:
- Stock and Standard Solutions: Prepare as described in the HPLC-DAD protocol.
- Sample Preparation: Due to the higher sensitivity of UHPLC, a higher dilution factor may be applicable. Follow a similar dilution scheme as the HPLC protocol, but ensure the final concentration is within the linear range and above the LOQ. Filter samples through a 0.22 µm membrane filter prior to injection to protect the UHPLC column and system.
Validation Steps:
- Follow ICH Q2(R1) guidelines as outlined in the HPLC protocol. The key differentiator will be the significantly reduced run time (3 minutes vs. 11 minutes), enabling a much faster throughput of samples during validation and routine analysis.
Decision Matrix and Workflow
The choice between HPLC-DAD and UHPLC-UV is multi-faceted. The following diagram and decision matrix integrate the key factors to guide the selection process.
Diagram 1: Technology Selection Workflow for Posaconazole Analysis
Table 3: Decision Matrix for Selecting HPLC-DAD vs. UHPLC-UV
| Decision Criterion | HPLC-DAD Recommendation | UHPLC-UV Recommendation | Remarks |
|---|---|---|---|
| Sample Throughput Need | Low to Moderate | High | UHPLC's 3-min run time [1] offers a ~4x throughput increase over HPLC (11 min). |
| Method Development Stage | Strongly Recommended | Less Suitable | DAD's spectral data is crucial for verifying peak purity and identifying co-eluting impurities during development [60]. |
| Available Budget | Lower Capital Cost | Higher Capital Cost | HPLC systems and consumables (e.g., 5µm columns) are generally less expensive than UHPLC equivalents. |
| Solvent Consumption & Waste | Higher (~16.5 mL/run) | Lower (~1.2 mL/run) | UHPLC's solvent economy aligns with Green Analytical Chemistry principles [1] [62]. |
| Data Integrity & Regulatory Needs | High (Spectral Confirmation) | Standard (Chromatographic) | Regulatory agencies increasingly expect spectral confirmation for peak purity, which DAD provides [60]. |
| Required Sensitivity | LOD: 0.82 µg/mL [1] | LOD: 1.04 µg/mL [1] | HPLC-DAD may have a slight edge in LOD for this specific method, but this is system-dependent. |
Concluding Remarks
The selection between HPLC-DAD and UHPLC-UV for posaconazole quantification is not a matter of one technology being universally superior, but rather of matching the tool to the task. HPLC-DAD is the prudent choice for routine quality control, method development, and environments where capital expenditure is a primary constraint and spectral confirmation of peak identity is valued. In contrast, UHPLC-UV is the superior tool for high-throughput laboratories where analytical speed, high resolution, and solvent economy are the driving factors.
This decision matrix provides a scientifically-grounded, practical framework to guide researchers and drug development professionals in making an informed, rational selection that optimizes resources and ensures data quality for their specific application in the quantitation of posaconazole.
Conclusion
The comparative analysis of HPLC-DAD and UHPLC-UV for posaconazole quantitation demonstrates that both are highly reliable and validated techniques, yet they serve distinct purposes. UHPLC-UV offers superior speed, reduced solvent consumption, and enhanced chromatographic resolution, making it ideal for high-throughput environments. In contrast, HPLC-DAD remains a robust, accessible, and cost-effective solution for routine quality control. The choice between them hinges on specific application needs, balancing analysis speed, operational costs, and available instrumentation. Future directions point towards wider adoption of UHPLC, integration with more sophisticated detection systems like tandem mass spectrometry, the application of green chemistry principles to minimize environmental impact, and the use of advanced data modeling to further optimize analytical methods for clinical and pharmaceutical research.
References
- 1. A Comparative Study of Newly Developed HPLC-DAD ... - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4167226/]
- 2. Advancing posaconazole quantification analysis with a new ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10314186/]
- 3. Development of an HPLC–UV method for quantification of ... [https://bmcchem.biomedcentral.com/articles/10.1186/s13065-024-01349-2]
- 4. High Performance Liquid Chromatographic Assay for the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4807048/]
- 5. Development and validation of an analytical method for the ... [https://www.sciencedirect.com/science/article/abs/pii/S0731708524006113]
- 6. How It Works: UV Detection for HPLC [https://www.chromatographyonline.com/view/how-it-works-uv-detection-hplc-0]
- 7. How HPLC Detectors Work [https://www.thermofisher.com/us/en/home/industrial/chromatography/chromatography-learning-center/liquid-chromatography-information/hplc-system-components/how-hplc-detectors-work.html]
- 8. Diode Array Detector HPLC | DAD [https://scioninstruments.com/us/blog/diode-array-detector-hplc/]
- 9. UHPLC, Part II: Benefits [https://www.chromatographyonline.com/view/uhplc-part-ii-benefits]
- 10. U-HPLC, UV/VIS or DAD (PDA) detector [http://www.chromforum.org/viewtopic.php?t=10457]
- 11. A Generic UHPLC-UV-MS Method for Cleaning Verification ... [https://www.americanpharmaceuticalreview.com/Featured-Articles/170906-A-Generic-UHPLC-UV-MS-Method-for-Cleaning-Verification-of-Highly-Potent-Drugs/]
- 12. A High-Performance Liquid Chromatography with ... [https://www.mdpi.com/2072-6643/15/18/4061]
- 13. Structural and spectroscopic properties of posaconazole [https://www.sciencedirect.com/science/article/abs/pii/S0022286018315072]
- 14. Posaconazole [https://en.wikipedia.org/wiki/Posaconazole]
- 15. Posaconazole CAS#: 171228-49-2 [https://www.chemicalbook.com/ProductChemicalPropertiesCB9946972_EN.htm]
- 16. Formation and Characterisation of Posaconazole Hydrate ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9862180/]
- 17. UV-Vis Spectroscopy: Principle, Parts, Uses, Limitations [https://microbenotes.com/uv-vis-spectroscopy/]
- 18. Ultraviolet Detectors: Perspectives, Principles, and Practices [https://www.chromatographyonline.com/view/ultraviolet-detectors-perspectives-principles-and-practices]
- 19. UV detector: Significance and symbolism [https://www.wisdomlib.org/concept/uv-detector]
- 20. Qualitative Applications of Ultraviolet Visible Absorption ... [https://chem.libretexts.org/Courses/Providence_College/CHM_331_Advanced_Analytical_Chemistry_1/09%3A_Applications_of_Ultraviolet-Visable_Molecular_Absorption_Spectrometry/9.03%3A_Qualitative_Applications_of_Ultraviolet_Visible_Absorption_Spectroscopy]
- 21. Advancing posaconazole quantification analysis with... [https://f1000research.com/articles/12-468]
- 22. Simultaneous quantification of voriconazole and ... [https://www.sciencedirect.com/science/article/pii/S1570023207000633]
- 23. Stability-Indicating HPLC Method for Posaconazole Bulk ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3383215/]
- 24. Development and validation of an HPLC-DAD method for ... [https://www.sciencedirect.com/science/article/pii/S1878535220304494]
- 25. Determination of Posaconazole in Plasma/Serum by High- ... [https://www.mdpi.com/2297-8739/4/2/16]
- 26. chromatographR: An introduction to HPLC-DAD analysis [https://ethanbass.github.io/chromatographR/articles/chromatographR.html]
- 27. Development and Validation of HPLC-DAD and UHPLC ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6151785/]
- 28. Myths in Ultrahigh-Pressure Liquid Chromatography [https://www.chromatographyonline.com/view/myths-ultrahigh-pressure-liquid-chromatography]
- 29. Recent innovations in UHPLC columns and instrumentation [https://www.sciencedirect.com/science/article/pii/S0165993614001939]
- 30. Advances in ultra‐high‐pressure and multi‐dimensional liquid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10989561/]
- 31. Development and Validation of a UHPLC UV Method for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4995228/]
- 32. Simultaneous Determination of Voriconazole and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2704663/]
- 33. Development, Validation, and Routine Application of a ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2916319/]
- 34. Validation of a method for itraconazole and major metabolite ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10529130/]
- 35. Simultaneous determination of itraconazole and its CYP3A4 ... [https://jphcs.biomedcentral.com/articles/10.1186/s40780-020-00167-7]
- 36. UV vs Diode-Array (PDA) Detectors for (U)HPLC [https://www.ssi.shimadzu.com/service-support/faq/liquid-chromatography/knowledge-base/uv-vs-pda-detectors/index.html]
- 37. Hplc-based bioanalytical method development and... [https://journals.innovareacademics.in/index.php/ijap/article/view/54731]
- 38. Important Guidelines for Optimizing Speed and Sensitivity ... [https://www.chromatographyonline.com/view/important-guidelines-optimizing-speed-and-sensitivity-small-molecule-lc-uv-and-lc-ms]
- 39. Real Solutions to Improve Your HPLC Peak Resolution [https://www.thermofisher.com/blog/analyteguru/real-solutions-to-improve-your-hplc-peak-resolution/]
- 40. Validation of an HPLC-DAD Method for Quercetin ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10748265/]
- 41. Development, Validation and Application of a Novel ... [https://www.mdpi.com/2297-8739/9/11/325]
- 42. Heterogeneous ensemble learning applied to UV-VIS ... [https://www.sciencedirect.com/science/article/abs/pii/S016974392500070X]
- 43. An eco-friendly chemometrics assisted UV spectrophotometric ... [https://bmcchem.biomedcentral.com/articles/10.1186/s13065-025-01528-9]
- 44. Optimization and validation of HPLC–DAD method for ... [https://link.springer.com/article/10.1007/s00217-023-04328-4]
- 45. Abstract [https://pubmed.ncbi.nlm.nih.gov/25258695/]
- 46. hplc-based bioanalytical method development and ... [https://www.academia.edu/143957450/HPLC_BASED_BIOANALYTICAL_METHOD_DEVELOPMENT_AND_VALIDATION_FOR_THE_ESTIMATION_OF_POSACONAZOLE_IN_SPIKED_RAT_PLASMA]
- 47. Compensate for or Minimize Matrix Effects? Strategies ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7412464/]
- 48. A Simple HPLC-DAD Method for the Therapeutic ... [https://www.mdpi.com/1420-3049/29/21/5039]
- 49. Matrix Effects on Quantitation in Liquid Chromatography [https://www.chromatographyonline.com/view/matrix-effects-on-quantitation-in-liquid-chromatography-sources-and-solutions]
- 50. Enhancing Sustainable Analytical Chemistry in Liquid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11243568/]
- 51. HPLC 2025 Preview: The Road To Sustainable Analytical ... [https://www.chromatographyonline.com/view/hplc-2025-preview-the-road-to-sustainable-analytical-chemistry]
- 52. Ecofriendly analytical quality by design-based HPLC ... [https://www.sciencedirect.com/science/article/abs/pii/S0026265X25033648]
- 53. Eco-Friendly AQbD-Driven HPLC: A Sustainable Approach ... [https://jyoungpharm.org/10.5530/jyp.20250087]
- 54. Green metric-based RP-HPLC method development and ... [https://link.springer.com/article/10.1007/s44371-025-00231-x]
- 55. An eco-friendly HPLC method for the sustainable analysis ... [https://pubs.rsc.org/en/content/articlelanding/2025/an/d5an00229j]
- 56. HPLC/UV or bioassay: two valid methods for posaconazole ... [https://pubmed.ncbi.nlm.nih.gov/22192527/]
- 57. Standardization and validation of a high-efficiency liquid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11223762/]
- 58. Development and Validation of HPLC-DAD and UHPLC ... [https://www.mdpi.com/1420-3049/22/9/1394]
- 59. Instrument contributions to resolution and sensitivity in ultra ... [https://www.sciencedirect.com/science/article/abs/pii/S0021967314018846]
- 60. "DAD vs. UV in HPLC: Why Results Differ" [https://www.linkedin.com/posts/sahar-sehati-604b42237_hplc-analyticalchemistry-pharmaceuticalanalysis-activity-7339641579034329088-b42K]
- 61. Comparison of Different Approaches for Calculating LOD ... [https://ps.tbzmed.ac.ir/Article/ps-40452]
- 62. Green and White Validated RP-HPLC Method for the ... [https://link.springer.com/article/10.1007/s40995-025-01928-5]