Research Articles
How to Validate Analytical Method Accuracy: A Lifecycle Guide for Scientists
This guide provides researchers and drug development professionals with a comprehensive framework for validating analytical method accuracy, a critical parameter for ensuring data reliability and regulatory compliance.
FTIR Spectroscopy: A Comprehensive Guide from Fundamentals to Cutting-Edge Applications in Biomedicine and Pharma
This article provides a comprehensive exploration of Fourier Transform Infrared (FTIR) spectroscopy, a versatile analytical technique renowned for its molecular fingerprinting capabilities.
A Comprehensive Guide to Method Comparison Experiments: Protocols, Guidelines, and Best Practices for Robust Research
This article provides a comprehensive framework for designing, executing, and validating method comparison experiments, a critical process in biomedical research and drug development.
A Practical Framework for Systematic Error Estimation in Method Comparison Experiments
This article provides a comprehensive guide for researchers and drug development professionals on estimating and correcting systematic error (bias) in method comparison studies.
Specific vs. Non-Specific Protein Quantification by UV-Vis: A Guide for Accurate Analysis in Biomedical Research
This article provides a comprehensive guide for researchers and drug development professionals on selecting and applying UV-Vis spectroscopy methods for protein quantification.
A Practical Guide to Developing Accurate UV-Vis Calibration Curves for Compound Quantification in Biomedical Research
This article provides a comprehensive guide for researchers and drug development professionals on developing and validating UV-Vis calibration curves for precise compound quantification.
Mastering UV-Vis Spectrometer Performance: A Comprehensive Guide to Alignment, Calibration, and Troubleshooting for Scientists
This article provides a complete guide for researchers and drug development professionals on ensuring optimal UV-Vis spectrometer performance.
Combating Contamination: A UV-Vis Spectroscopy Guide for Identification, Prevention, and Method Validation
This article provides a comprehensive guide for researchers and drug development professionals on leveraging UV-Vis spectroscopy for contamination control.
UV-Vis Spectroscopy for Reaction Kinetics: Advanced Monitoring in Pharmaceutical and Biomedical Research
This comprehensive article explores UV-Vis spectroscopy as a powerful analytical technique for monitoring chemical reaction kinetics, tailored for researchers, scientists, and drug development professionals.
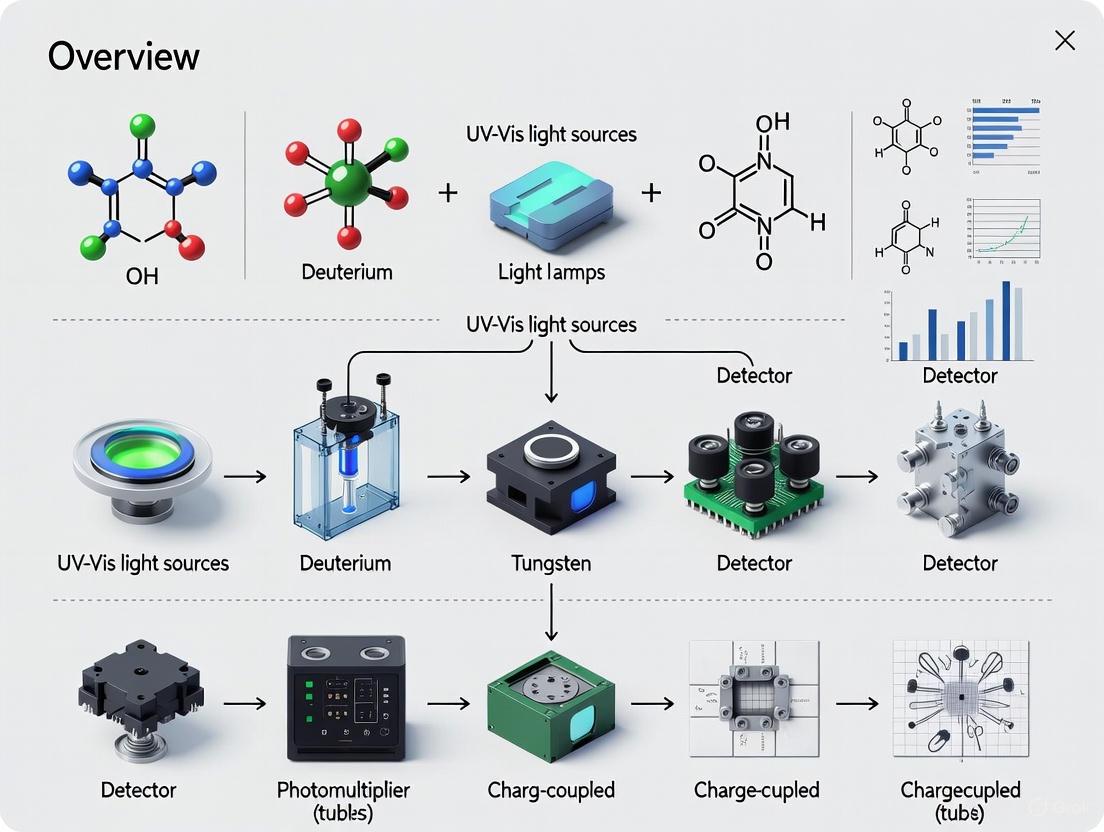
UV-Vis Light Sources and Detectors: A 2025 Technology Overview for Biomedical Research
This article provides a comprehensive overview of the fundamental principles, current technologies, and practical applications of UV-Vis light sources and detectors, tailored for researchers and drug development professionals.